Preparation method of dynamic pH response chain based on boric acid type metal organic complex
A metal-organic and boric acid-based technology, applied in the field of preparation of biomedical functional materials, can solve the problems of few reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
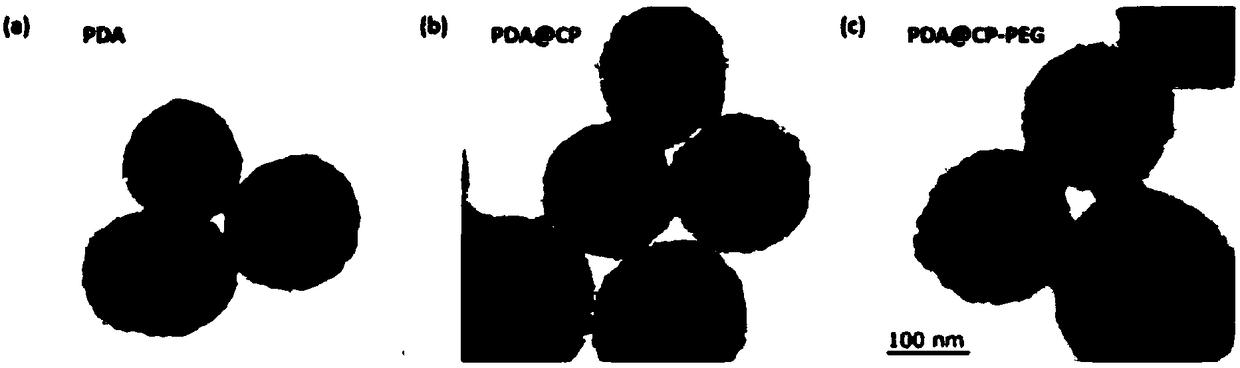
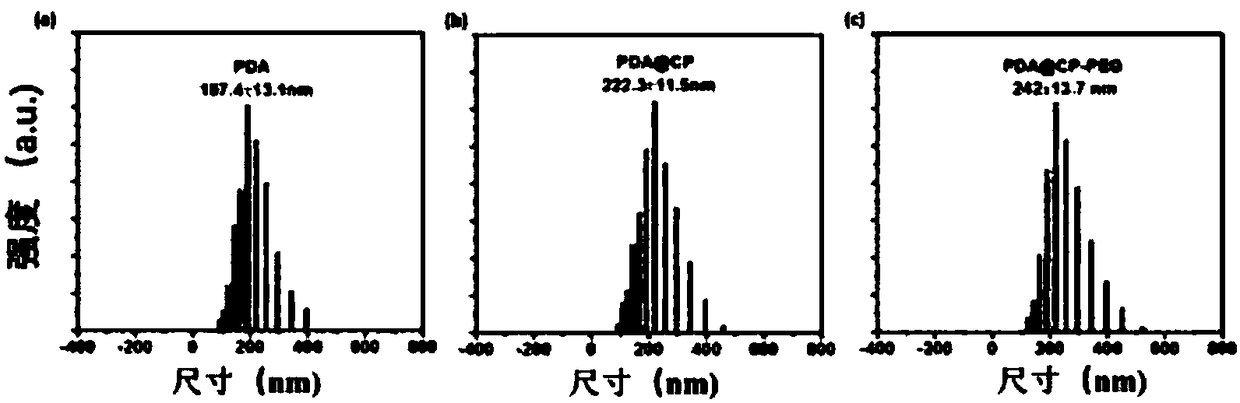
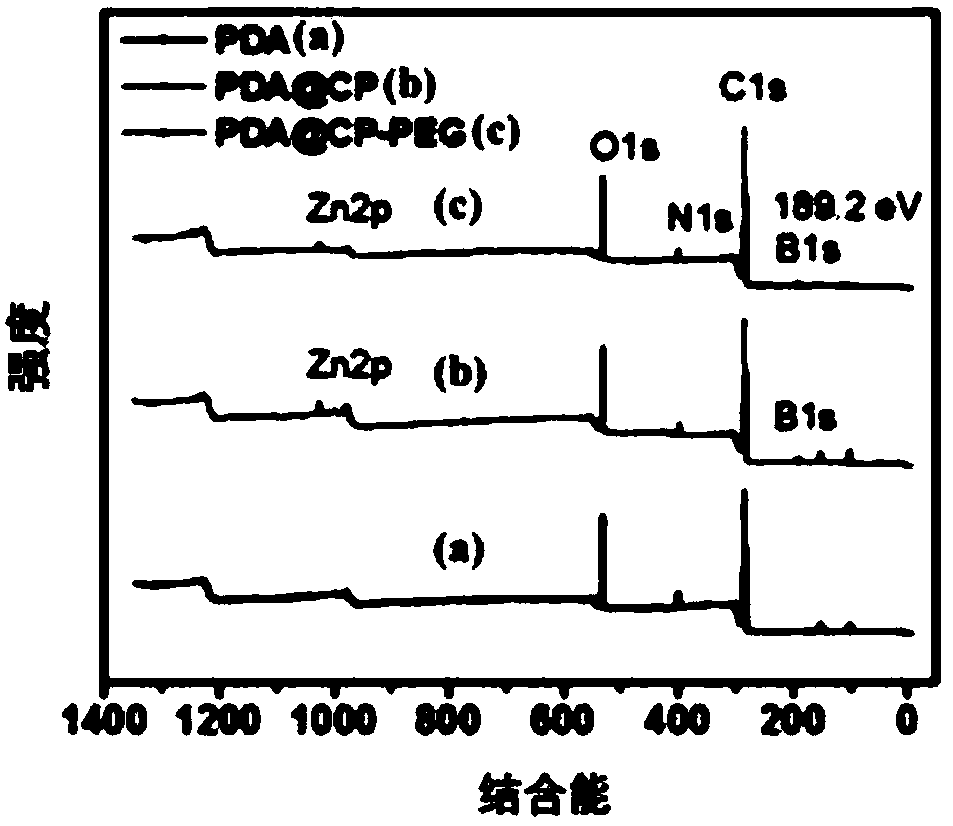
[0034]First 20mL of ethanol and 20mL of distilled water were mixed, then 0.4g of dopamine hydrochloride was added, and stirred and dispersed with a magnet for 20min; the obtained solution was slowly added with 1mL of ammonia solution (28%-30% ), the solution immediately changed from yellow to dark brown and continuously stirred for 12 hours, and was washed with distilled water several times, and dried for 12 hours at 50 degrees to prepare polydopamine nanoparticles (PDA); then the prepared 80mgPDA , 40mg of zinc nitrate hexahydrate, 10mL of absolute ethanol, and 10mL of N,N-dimethylformamide (DMF) were added to the reaction ax of polytetrafluoroethylene, then 7mg of BBDC and 7mg of BTC were added, and in Hydrothermal polymerization in a blast drying oven at 100°C for 6 hours, then cooled to room temperature, washed with distilled water several times, and finally dried in vacuum at 50°C, and then repeated the above steps 3 times to prepare PDA@CP. Then the obtained PDA@CP was a...
Embodiment 2
[0036] First 25mL of ethanol and 25mL of distilled water were mixed, then 0.5g of dopamine hydrochloride was added, and stirred and dispersed with a magnet for 30min; the resulting solution was slowly added to 2mL of ammonia solution (28%-30% ), the solution immediately changed from yellow to dark brown and continuously stirred for 24 hours, and was washed with distilled water several times, and dried at 60 degrees for 24 hours to prepare polydopamine nanoparticles (PDA); then the prepared 100mgPDA , 50mg of zinc nitrate hexahydrate, 12.5mL of absolute ethanol, and 12.5mL of N,N-dimethylformamide (DMF) were added to the polytetrafluoroethylene reaction ax, and then 8mg of BBDC and 8mg of BTC were added, And hydrothermally polymerized in a blast drying oven at 120°C for 12 hours, then cooled to room temperature, washed with distilled water several times, and finally dried in vacuum at 60°C, and then repeated the above steps 3 times to prepare PDA@CP. Then the obtained PDA@CP wa...
Embodiment 3
[0038] First 30mL of ethanol and 30mL of distilled water were mixed, then 0.6g of dopamine hydrochloride was added, and stirred and dispersed with a magnet for 40min; the resulting solution was slowly added to 3mL of ammonia solution (28%-30% ), the solution immediately changed from yellow to dark brown and continuously stirred for 48 hours, and was washed with distilled water several times, and dried for 48 hours at 70 degrees to prepare polydopamine nanoparticles (PDA); then the prepared 120mgPDA , 60mg of zinc nitrate hexahydrate, 15mL of absolute ethanol, and 15mL of N,N-dimethylformamide (DMF) were added to the polytetrafluoroethylene reaction ax, then 9mg of BBDC and 9mg of BTC were added, and in Hydrothermal polymerization was carried out at 140°C for 24 hours in a blast drying oven, then cooled to room temperature, washed with distilled water several times, and finally dried in vacuum at 60°C, and then the above steps were repeated 3 times to prepare PDA@CP. Then the o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com