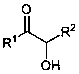
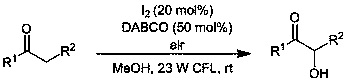Green preparation method of alpha-hydroxyketone
A green technology for hydroxyketones, applied in the field of green preparation of α-hydroxyketones, to achieve the effects of wide application of substrates, simple and easy method, convenient and safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] Preparation method of the present invention can represent with following typical reaction formula:
[0018]
Embodiment 1
[0019] Example 1 A green preparation method of α-hydroxy ketone, the method refers to sequentially adding 0.3 mmol of 1-phenyl-1-propanone, 0.06 mmol of iodine, 1,4-diazabicyclo[2.2.2]octane Add 0.15 mmol of alkane (DABCO) and 2.0 mL of methanol into a 5 mL glass reaction vial; then, under the irradiation of a 23 W compact fluorescent lamp (CFL), stir the reaction at room temperature for 24 h in an air atmosphere to obtain a reaction mixture. Separation by silica gel column chromatography yielded 34.5 mg of 1-phenyl-2-hydroxyl-1-propanone, whose structural formula is as follows:
[0020] .
[0021] Among them: the mobile phase used in the silica gel column chromatography separation is a mixed solution obtained by mixing PE and EtOAc at a ratio of 10 mL:1 mL.
[0022] The analysis results of the obtained product 1-phenyl-2-hydroxy-1-propanone are as follows: colorless liquid; yield 77%. 1 H NMR (600 MHz, CDCl 3 ): δ 7.92 (d, J = 8.4 Hz, 2H), 7.61 (t, J = 7.2 Hz, 1H), 7...
Embodiment 2
[0023] Example 2 A green preparation method of α-hydroxy ketone, the method refers to sequentially adding 0.3 mmol of 1-p-tolyl-1-propanone, 0.06 mmol of iodine, 1,4-diazabicyclo[2.2.2] Octane 0.15 mmol (DABCO) and methanol 2.0 mL were added to a 5 mL glass reaction bottle; then under the irradiation of a 23 W compact fluorescent lamp (CFL), the reaction was stirred at room temperature in an air atmosphere for 27 h to obtain a reaction mixture, which was subjected to Silica gel column chromatography to obtain 30.5 mg of 1-p-tolyl-2-hydroxyl-1-propanone, whose structural formula is as follows:
[0024] .
[0025] Among them: the mobile phase used in the silica gel column chromatography separation is a mixed solution obtained by mixing PE and EtOAc at a ratio of 15 mL:1 mL.
[0026] The analysis results of the obtained product 1-p-tolyl-2-hydroxyl-1-propanone are as follows: light yellow liquid, yield 62%. 1 HNMR (600 MHz, CDCl 3 ): δ 7.82 (d, J = 8.4 Hz, 2H), 7.29 (d, J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com