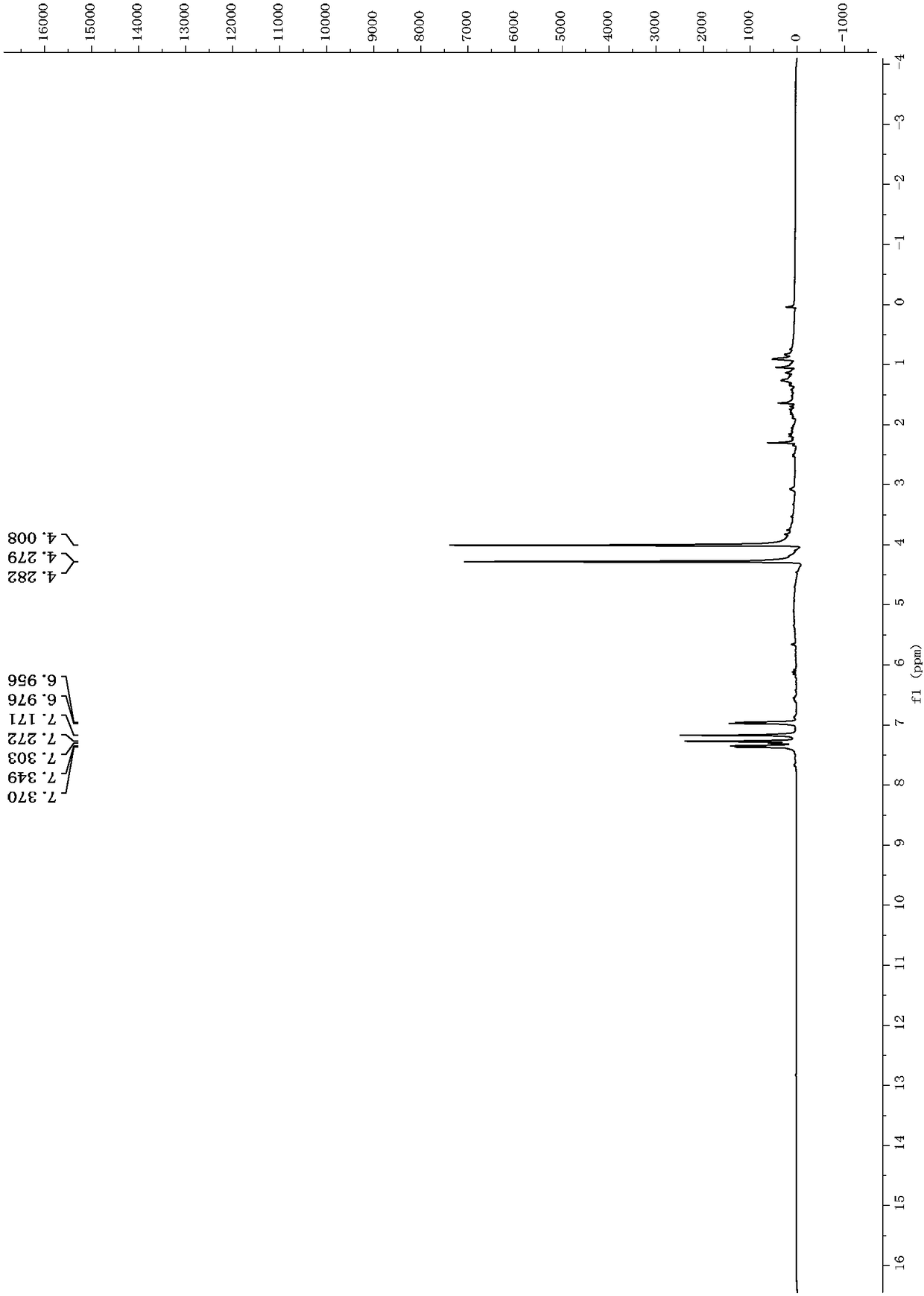
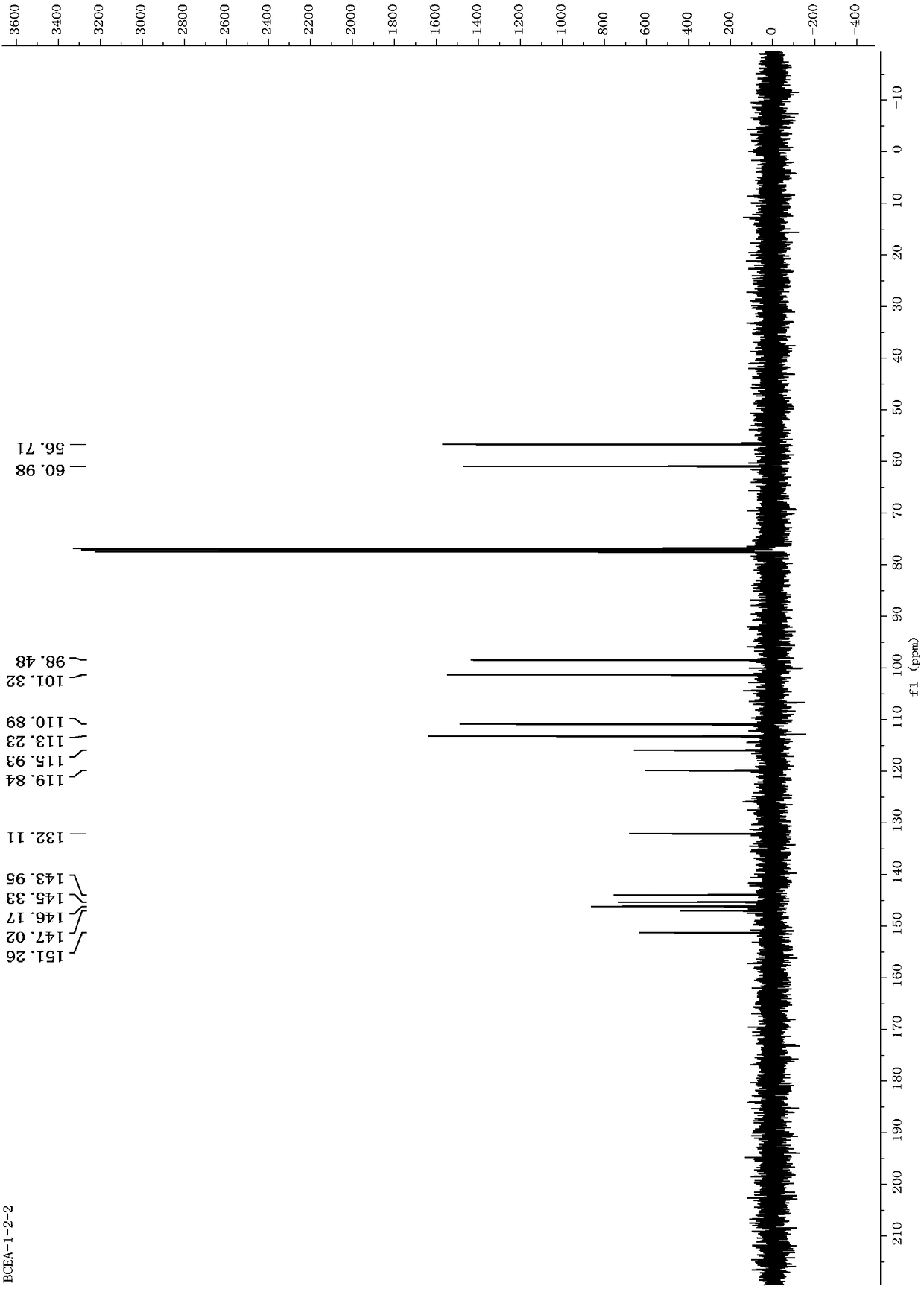
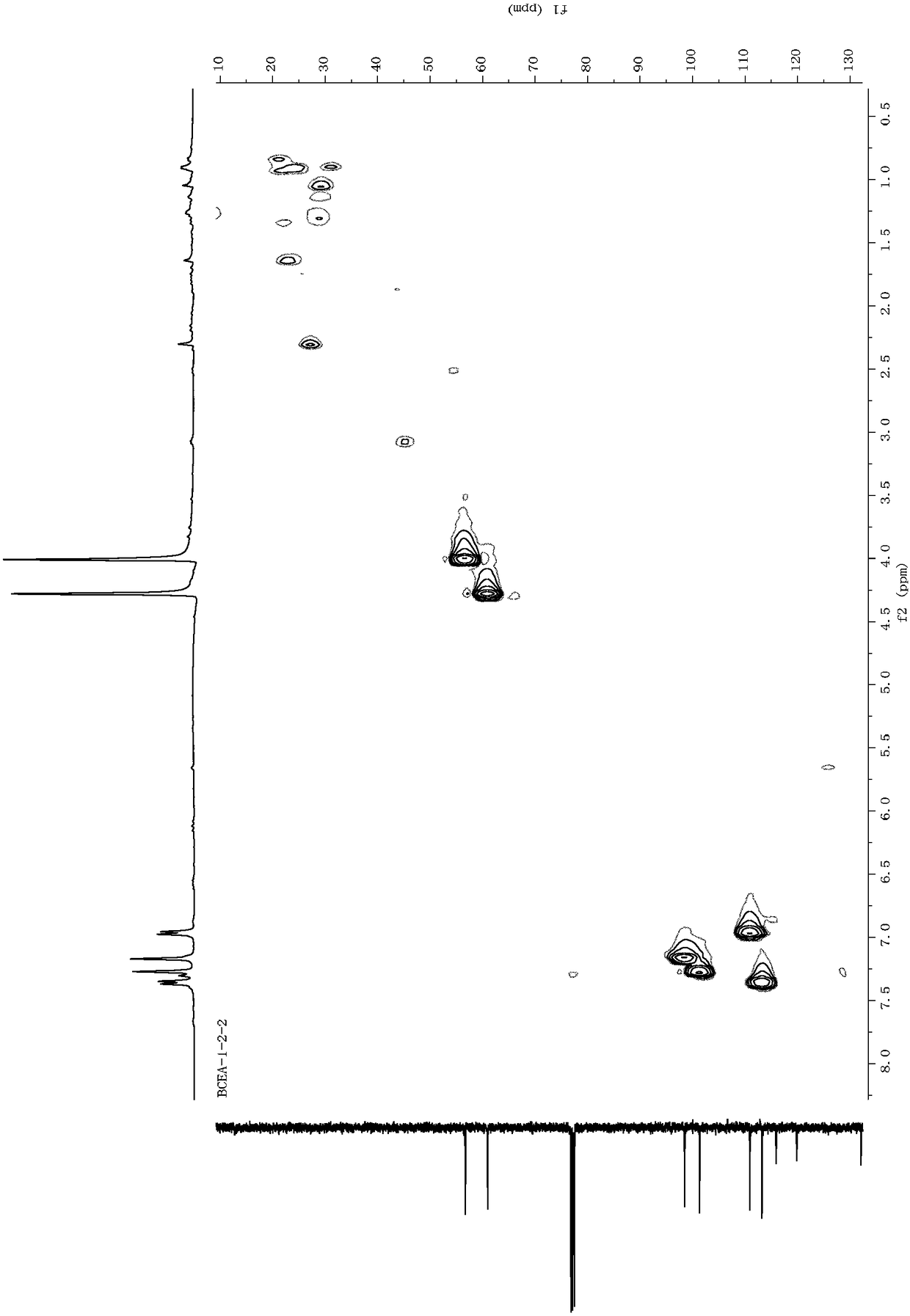
Dibenzofuran derivative, and preparation method and application thereof
A compound and extract technology, applied in the field of dibenzofuran derivatives and its preparation, can solve the problems of insufficient research and development and utilization, and achieve good anti-rheumatoid arthritis effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The preparation of embodiment 1 compound of the present invention
[0085] 1. Extraction
[0086] (1) Take 63 kg of rhizomes of Tengsu chinensis, crush them into coarse powder, soak them in 120 L of 90% ethanol solution, and extract them by cold soaking for 3 times, each time for 3 days. The extracts were combined and concentrated under reduced pressure to obtain 2650 g of the ethanol extract of Suteng.
[0087] (2) Disperse and mix 2650 g of the ethanol extract extract with 8 L of water, extract with petroleum ether and ethyl acetate in turn, concentrate under reduced pressure and dry in vacuo to obtain the extract of ethyl acetate (435.0 g).
[0088] 2. Separation and purification of compounds
[0089] Take 435.0 g of the extract from ethyl acetate, separate and purify it by silica gel column chromatography (100-200 mesh), mix and mix the sample, and elute with chloroform. The gradient elution procedure is shown in Table 1.
[0090] Table 1 Gradient elution program...
experiment example 1
[0107] Experimental Example 1 Anti-rheumatoid arthritis activity
[0108] The proliferation inhibitory activity of compounds on synoviocytes was detected by MTT assay. Synoviocytes were cultured in RPMI 1640 medium (containing 10% fetal bovine serum, penicillin and streptomycin at a concentration of 100 μg / mL, and L-glutamine at a concentration of 1 mmol / L), at 37°C and 5% CO 2 Incubate in a saturated humidity incubator until logarithmic growth phase. The synoviocytes in the logarithmic growth phase were inoculated into 96-well plates (the cell concentration was 1x105 / mL). After the cells adhered to the wall for 13 hours, 100 μL each of 9 test samples with different concentration gradients and the positive control drug methotrexate were added, and an equal volume of administration medium was added to the negative control group. After co-incubating for 48 h, 10 μL of MTT solution (5 mg / mL) was added to each well, and the supernatant was aspirated after continuing to culture f...
Embodiment 2
[0110] Example 2 Pharmacodynamic Research on Anti-rheumatoid Arthritis
[0111] Animals: SPF grade healthy rats, Wistar, male, six weeks old, weighing 180-200 g, purchased from Shanghai Slack Experimental Animal Co., Ltd. [certificate number: SCXK (Shanghai) 2012-0002]. The experimental animal center of Fujian University of Traditional Chinese Medicine was fed in an SPF-level laboratory with a light / dark cycle of 12 / 12h, a temperature of 25.0±0.5°C, and a relative humidity of 50%-70%.
[0112] 1. Establishment of bovine type II collagen-induced arthritis rat (CIA) model
[0113] Specific modeling method: Bovine type II collagen (2 mg / mL) and complete Freund's adjuvant (CFA) were mixed in equal volumes, and ground to make collagen emulsion. Subcutaneously inject collagen emulsion 0.2mL / rat at the base of the tail at 2cm, and inject at the base of the rat tail at 3cm on the 7th day (mixed emulsion of bovine type II collagen and incomplete Freund's adjuvant (IFA) equal volume) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com