Gene therapy medicament for treating hyperuricemia
A gene drug, uric acid technology, applied in gene therapy, drug combination, genetic engineering, etc., can solve the problem of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 Plasmid vector construction
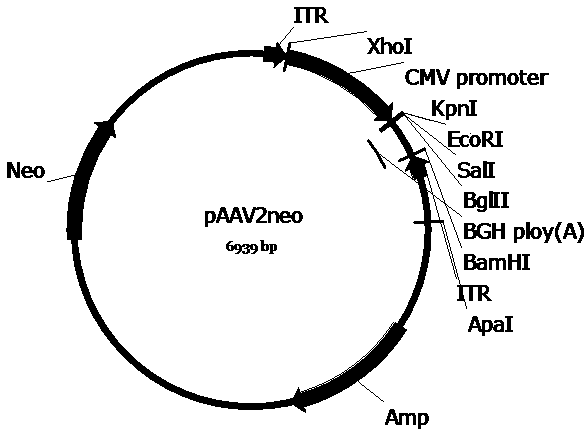
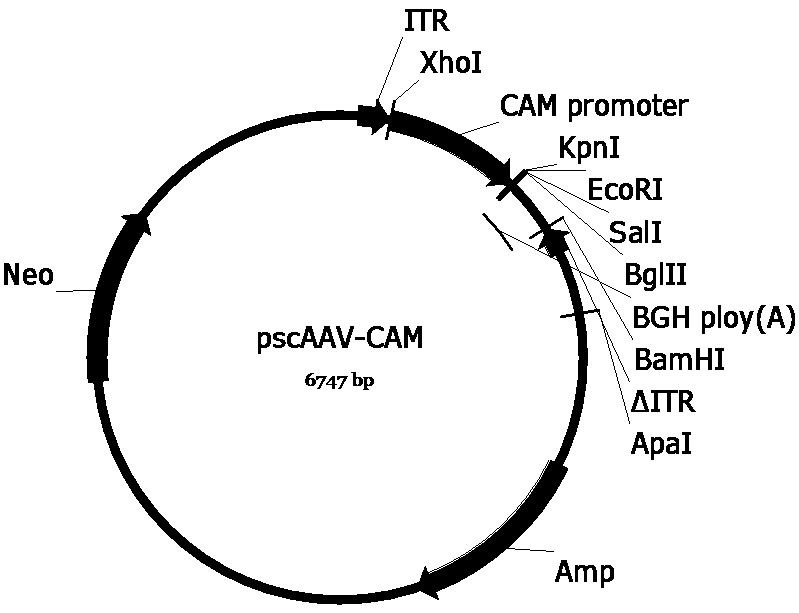
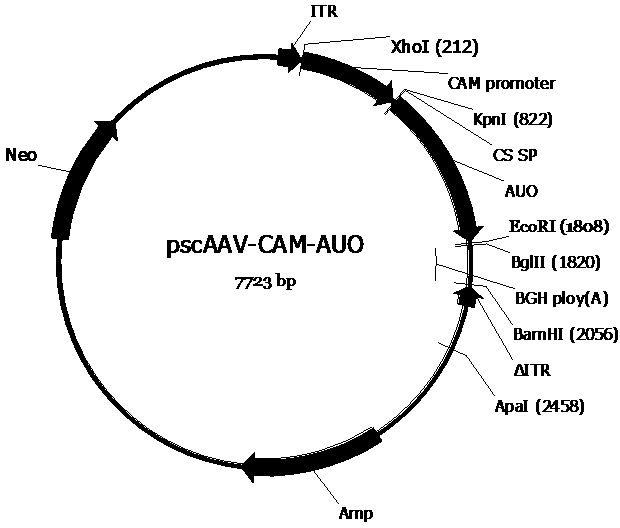
[0063] In order to construct the pscAAV-CAM-AUO and pscAAV-CAM-AUO-142T plasmids required for packaging recombinant AAV viruses, we first replaced the pAAV2neo with the self-designed CAM promoter (SEQ ID No.6) based on the company’s preserved pAAV2neo For the CMV promoter in the pAAV2neo vector, replace one side of the ITR sequence in the pAAV2neo vector with a mutated ITR sequence (named ΔITR) (SEQ ID No.8) that deletes the trs (terminal resolution site) and D sequences in the ITR of AAV2 , to obtain the pscAAV-CAM vector. Next, artificially synthesized CS SP-AUO (SEQ ID No.5) and CS SP-AUO-142T (SEQ ID No.9) (CS SP-AUO plus four tandem miR-142-3p target sequences , between the two are connected by EcoRI restriction site) sequences were cloned into the pscAAV-CAM vector between KpnI and EcoRI and KpnI and BglII restriction sites, respectively, to obtain pscAAV-CAM-AUO and pscAAV-CAM-AUO-142T carrier.
[0064] (1) Construction ...
Embodiment 2
[0070] Example 2 Preparation and assay of recombinant AAV virus
[0071] References (Xiao X, et al . J Virol. 1998; 72(3): 2224-2232.), using a three-plasmid packaging system to package recombinant AAV virus, and using cesium chloride density gradient centrifugation to separate, purify and package AAV virus. Briefly, the AAV vector plasmid (pscAAV-CAM-AUO or pscAAV-CAM-AUO-142T), the helper plasmid (pHelper), and the AAV Rep and Cap protein expression plasmids (pAAV-R2C1, pAAV-RC, pAAV-R2C8 or pAAV -R2C9) mixed according to the molar ratio of 1:1:1, transfect HEK293 cells by calcium phosphate method, after 48 hours of transfection, harvest the cells and culture supernatant, and use cesium chloride density gradient centrifugation to separate and purify the recombinant AAV virus . Packaged and purified to obtain scAAV1-CAM-AUO, scAAV1-CAM-AUO-142T, scAAV2-CAM-AUO, scAAV2-CAM-AUO-142T, scAAV8-CAM-AUO, scAAV8-CAM-AUO-142T, scAAV9-CAM-AUO and scAAV9-CAM-AUO-142T and other 8 kind...
Embodiment 3
[0077] Example 3 Establishment of mouse hyperuricemia model
[0078] References (Wu X, et al . Proc Natl Acad Sci USA. 1994; 91(2): 742-746.), to establish a mouse model of hyperuricemia. The homologous recombination method is used to knock out the urate oxidase gene in mouse embryonic stem cells to prepare transgenic mice heterozygous for the urate oxidase gene. The preparation of transgenic mice was completed by Beijing Biocytogen Biotechnology Co., Ltd. The urate oxidase gene heterozygous mouse C57BL / 6J prepared by Beijing Biocytogen Biotechnology Co., Ltd. + / - Transferred to Beijing Wujiahe Institute of Molecular Medicine Co., Ltd. Mice heterozygous for the urate oxidase gene appeared normal. Male and female C57BL / 6J heterozygous for the urate oxidase gene + / - Mating to obtain homozygous mice C57BL / 6J lacking urate oxidase gene - / - , as a model of hyperuricemia. Screening to identify male and female C57BL / 6J by measuring mouse phenotype + / - Homozygous mice C57BL / 6J...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com