Implants having a drug load of an oxysterol and methods of use
An oxidized sterol and implant technology, applied in the field of ductile implants and anti-compression implants, can solve problems such as bone defects that cannot match the shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0239] A first formulation of implants having the composition shown in Table 1 was prepared.
[0240] Table 1
[0241] CMC%
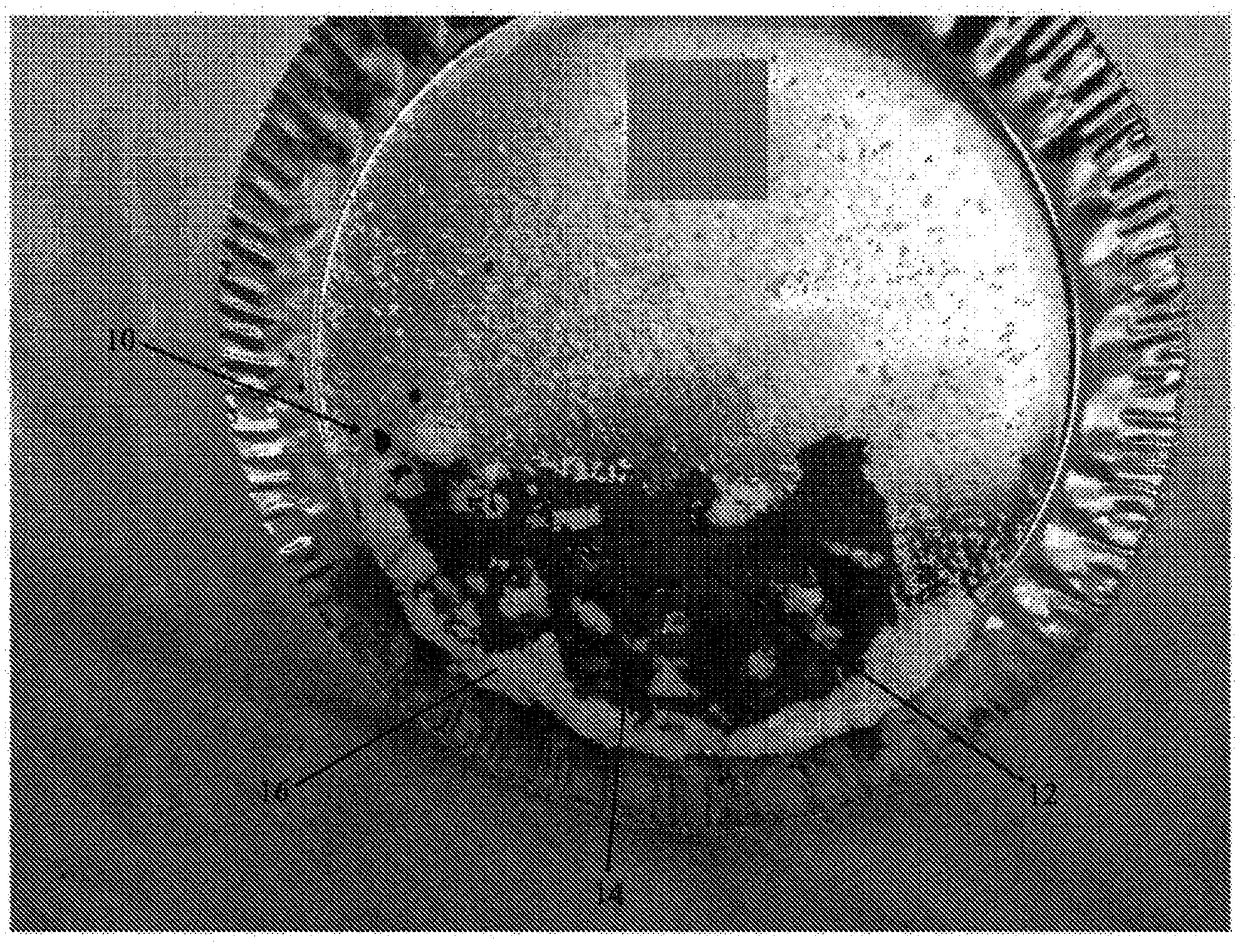
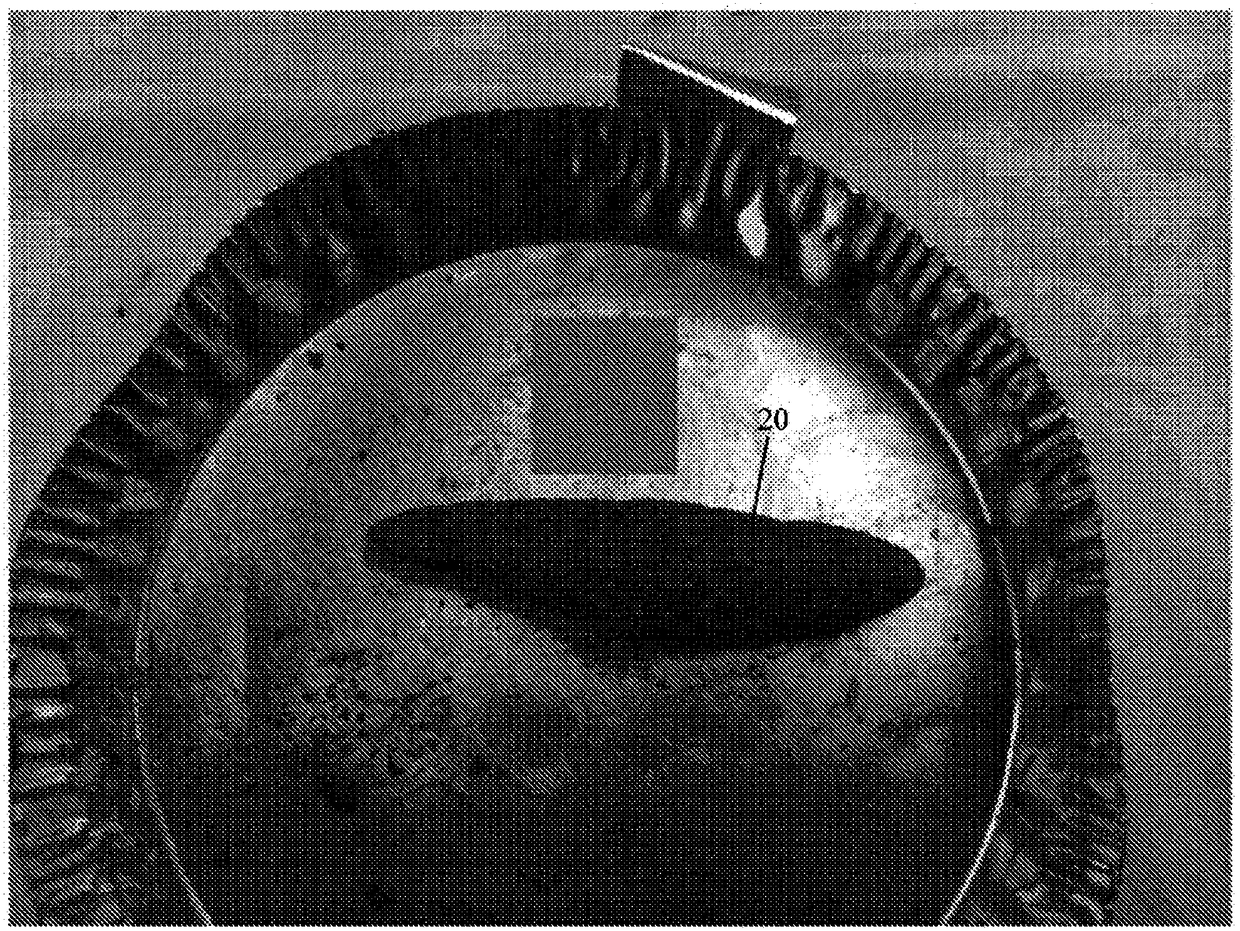
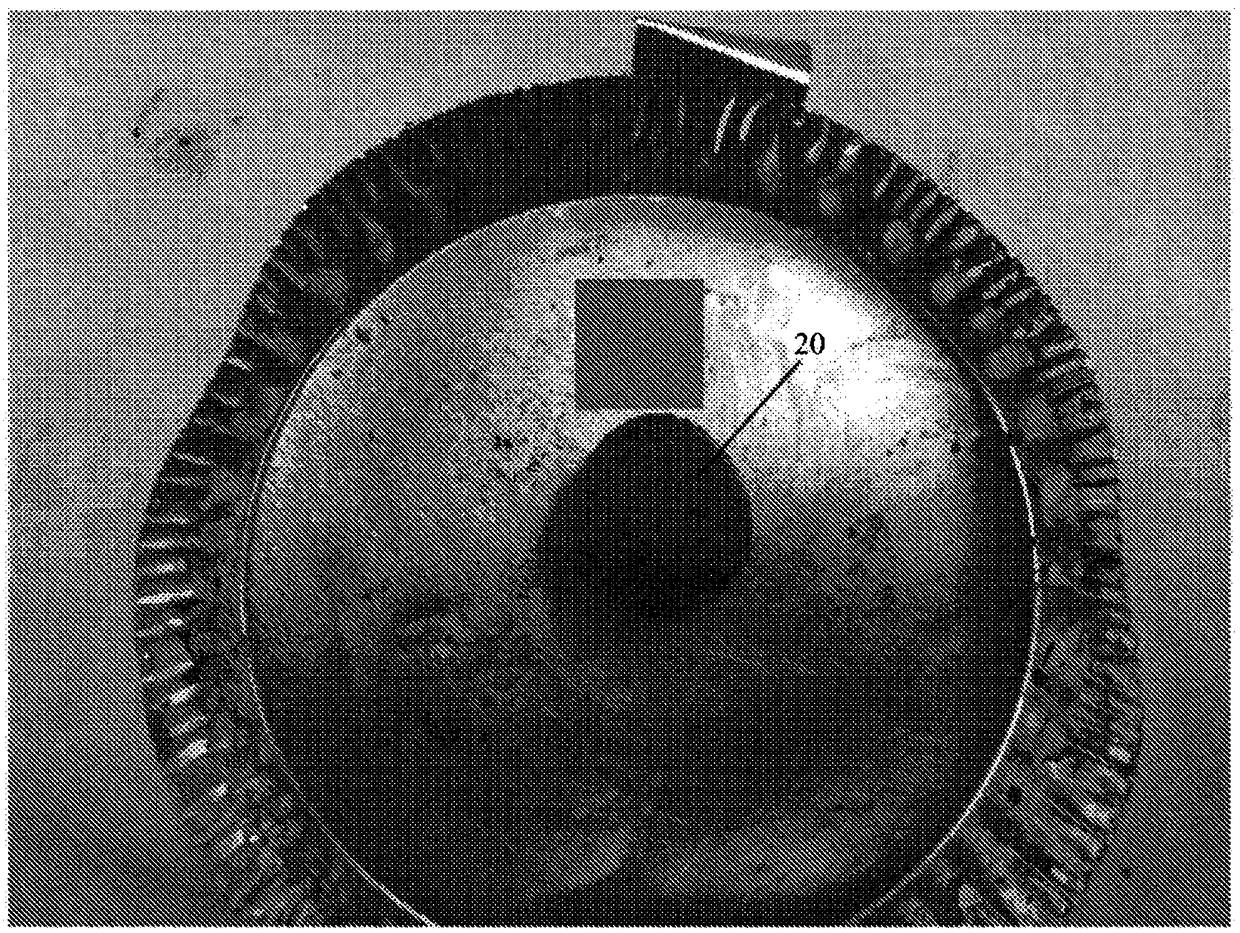
[0242] The values listed in Table 2 refer to the composition of the dry powder components in the matrix before wetting with physiologically acceptable saline. Carboxymethylcellulose is added to impart adhesive properties to the implant. Carboxymethylcellulose has also been found to cause the implant to expand significantly after wetting. In some cases, carboxymethylcellulose allowed the implant to expand in some dimensions to twice the size of the dry compound. The collagen added to the composition is porcine type I fibrotic collagen. The ceramic contains beta tricalcium phosphate and hydroxyapatite in a ratio of 85:15. The ceramic particles have a particle size of about 125 μm to about 750 μm. Small particles are preferred to create a larger surface area to volume ratio to achieve faster absorption after implantation in a bone defect. ...
example 2
[0244] Formulations of moldable implants having the composition shown in Table 2 were prepared.
[0245] Table 2
[0246] Collagen%
[0247] The values listed in Table 2 refer to the composition of the powder components of the matrix before wetting with physiologically acceptable saline. After wetting, the collagen was found to hold the implant together with little or no cross-linking. The collagen added to the composition is porcine type I fibrotic collagen. The ceramic contains beta tricalcium phosphate and hydroxyapatite in a ratio of 85:15. The ceramic particles have a particle size of about 125 μm to about 750 μm. Small particles are preferred to create a larger surface area to volume ratio to achieve faster absorption after implantation in a bone defect. A relatively high amount of ceramic is provided in the composition to impart compression resistance properties. Oxy133 was added to the composition at a level of about 400 mg / cc. Oxy133 was added in the...
example 3
[0249] Compositions were prepared according to the specifications of Example 1 or 2 above. Moisten the composition with sterile water.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com