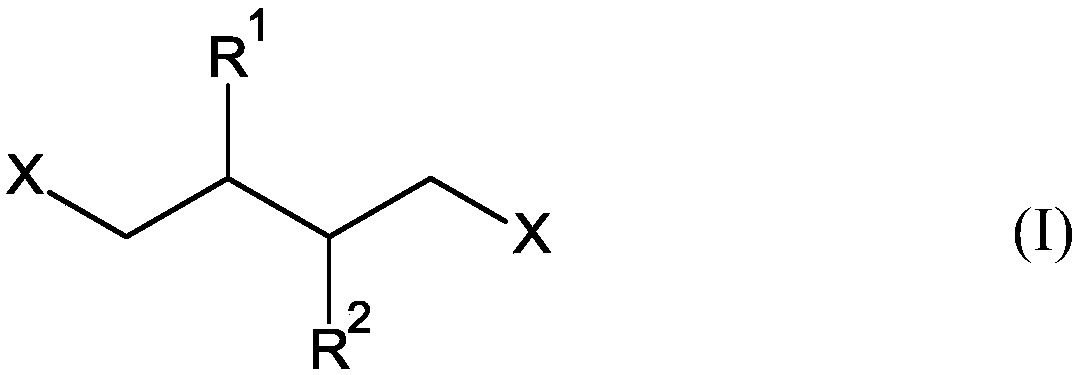
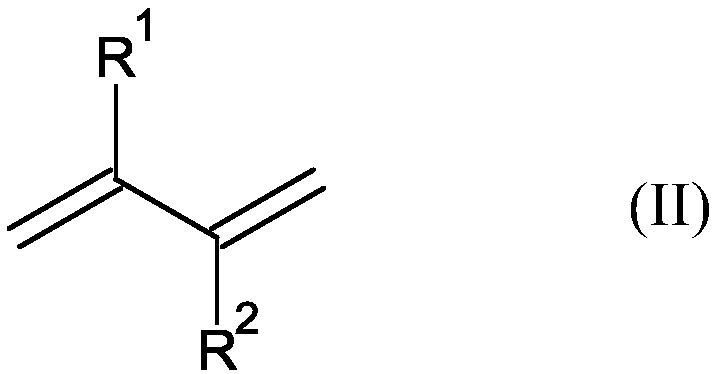
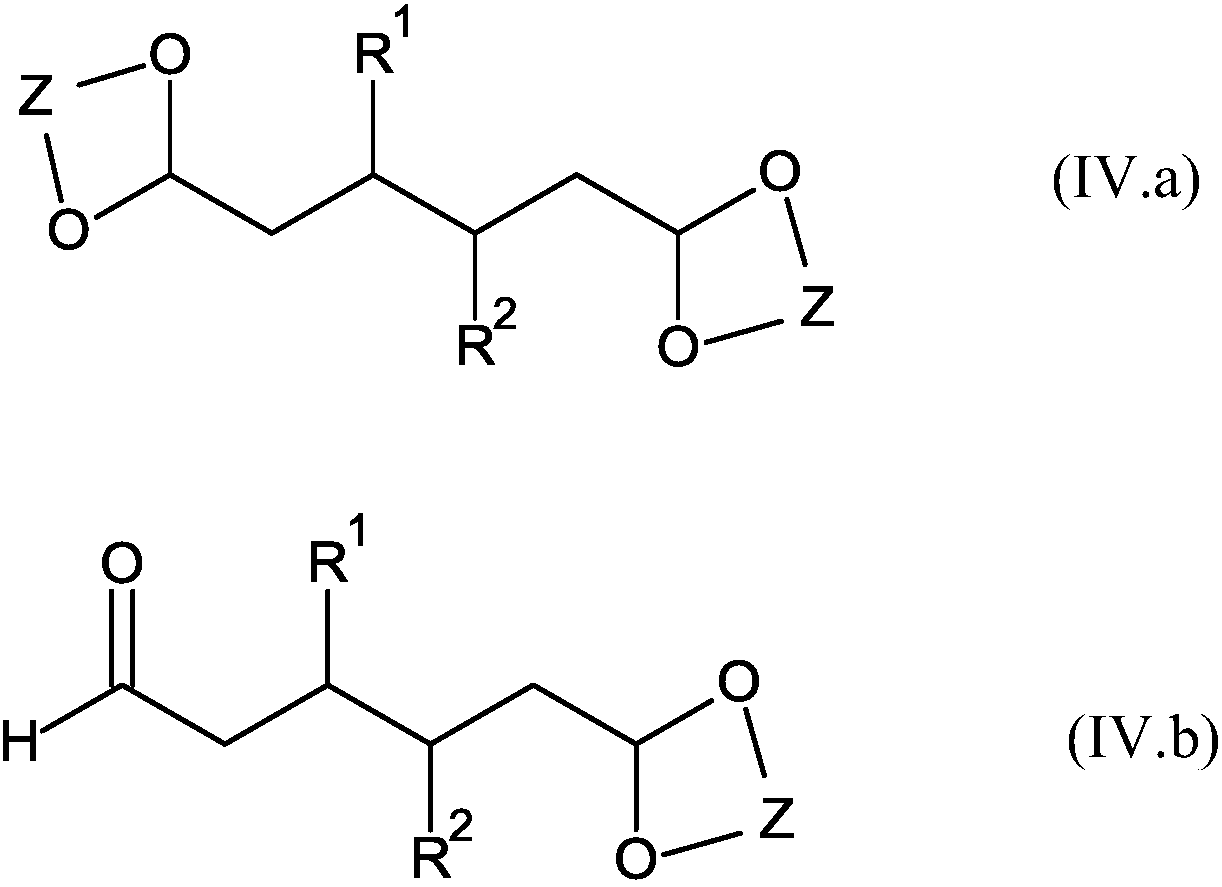
Hydroformylation process for producing 1,6-disubstituted hexane derivatives
A technology of hydroformyl and alkyl, applied in the field of producing polyamide 6.6
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0255] This example was performed using tMe-Ruca phosphite (compound (V.a)) according to the general procedure described above. The autoclave was heated to 80 °C and the reaction mixture was stirred at this temperature for 2 hours. The temperature was raised to 120°C and the reaction mixture was stirred at this temperature for 18 hours. Afterwards, gas chromatographic analysis showed 63% diacetals of 1,6-adipaldehyde, 16% diacetals of 1,2-, 1,3- and 1,4-hexanedial, 14% valeraldehyde acetal, 6% acetal of 3-pentenal and a small amount of other products. The ratio of the diacetals of 1,6-hexanedial to the diacetals of 1,2-, 1,3- and 1,4-hexanedial was 3.9.
Embodiment 2
[0257] This example was carried out according to example 1, except that Biphephos (compound (V.b)) was used instead of tMe-Ruca phosphite (compound (V.a)). Afterwards, gas chromatographic analysis showed 65% of the diacetals of 1,6-hexanedial, 9% of the diacetals of 1,2-, 1,3- and 1,4-hexanedial, 31% of the Acetals of aldehydes and a small amount of other products. The ratio of the diacetals of 1,6-hexanedial to the diacetals of 1,2-, 1,3- and 1,4-hexanedial was 7.2.
Embodiment 3
[0259] This example was performed using tMe-Ruca phosphite (compound (V.a)) according to the general procedure described above. The autoclave was heated to 80 °C and the reaction mixture was stirred at this temperature for 2 hours. The autoclave was cooled to 0 °C by means of an ice bath, and the pressure in the autoclave was reduced to 10 bar. The autoclave was heated to 120° C., and the reaction mixture was stirred at this temperature for 18 hours. Afterwards, gas chromatographic analysis showed 73% of the diacetals of 1,6-hexanedial, 12% of the diacetals of 1,2-, 1,3- and 1,4-hexanedial, 19% of the Acetals of aldehydes and a small amount of other products. The ratio of the diacetals of 1,6-hexanedial to the diacetals of 1,2-, 1,3- and 1,4-hexanedial was 6.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com