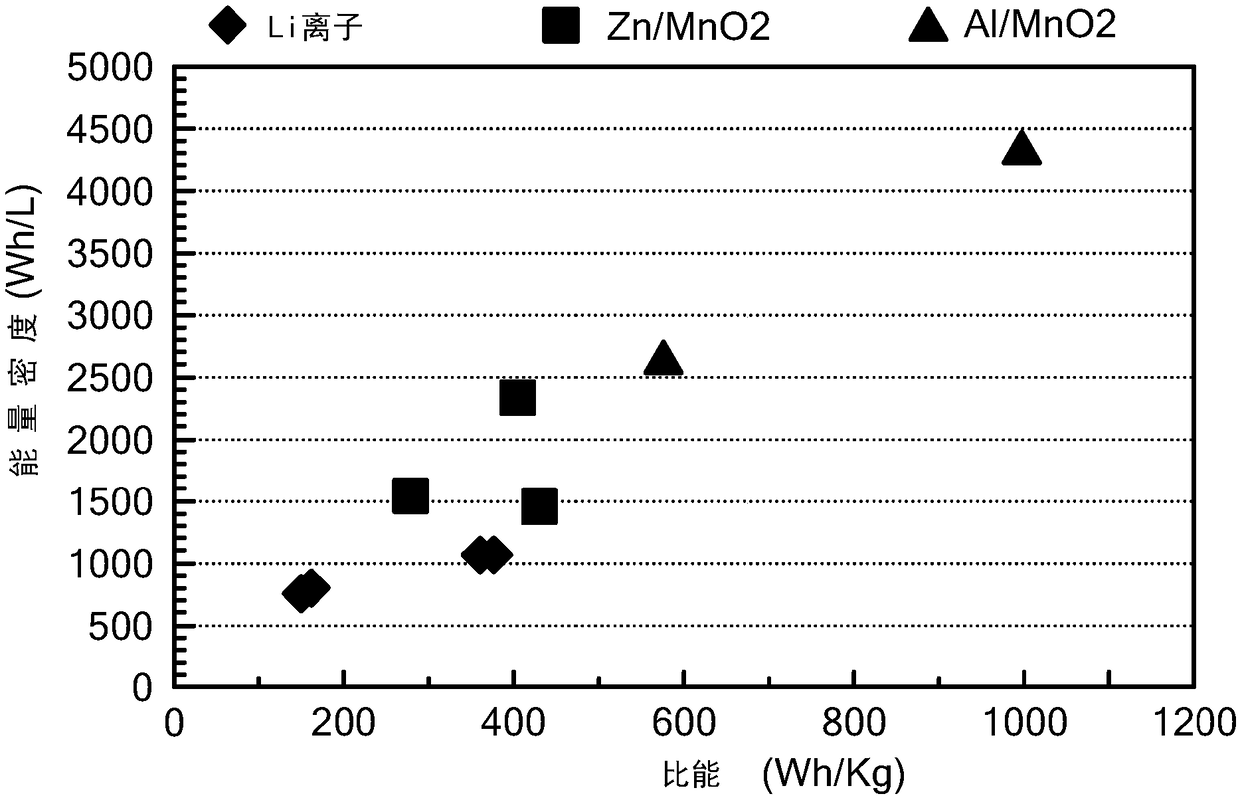
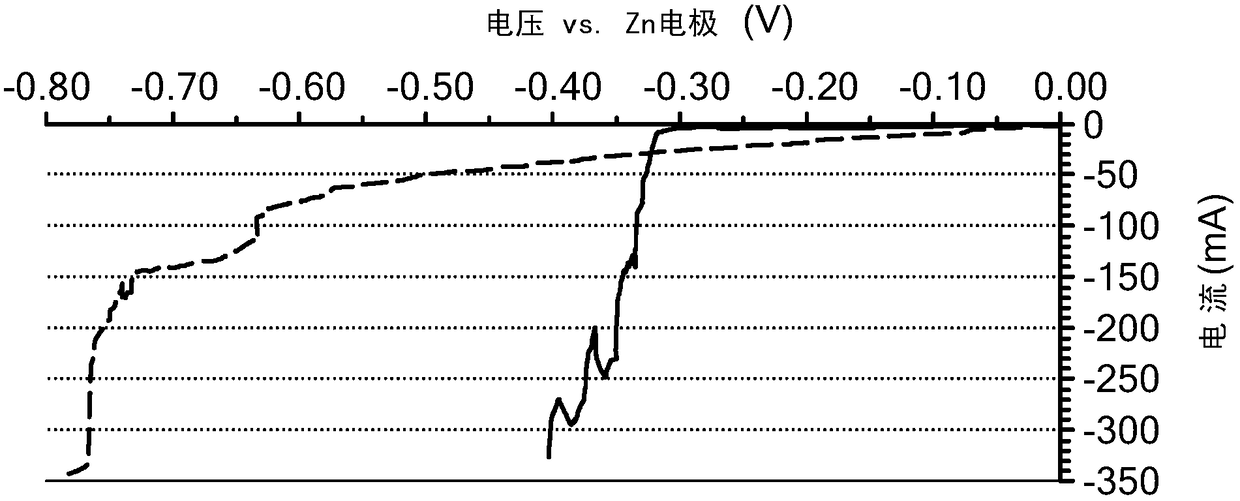
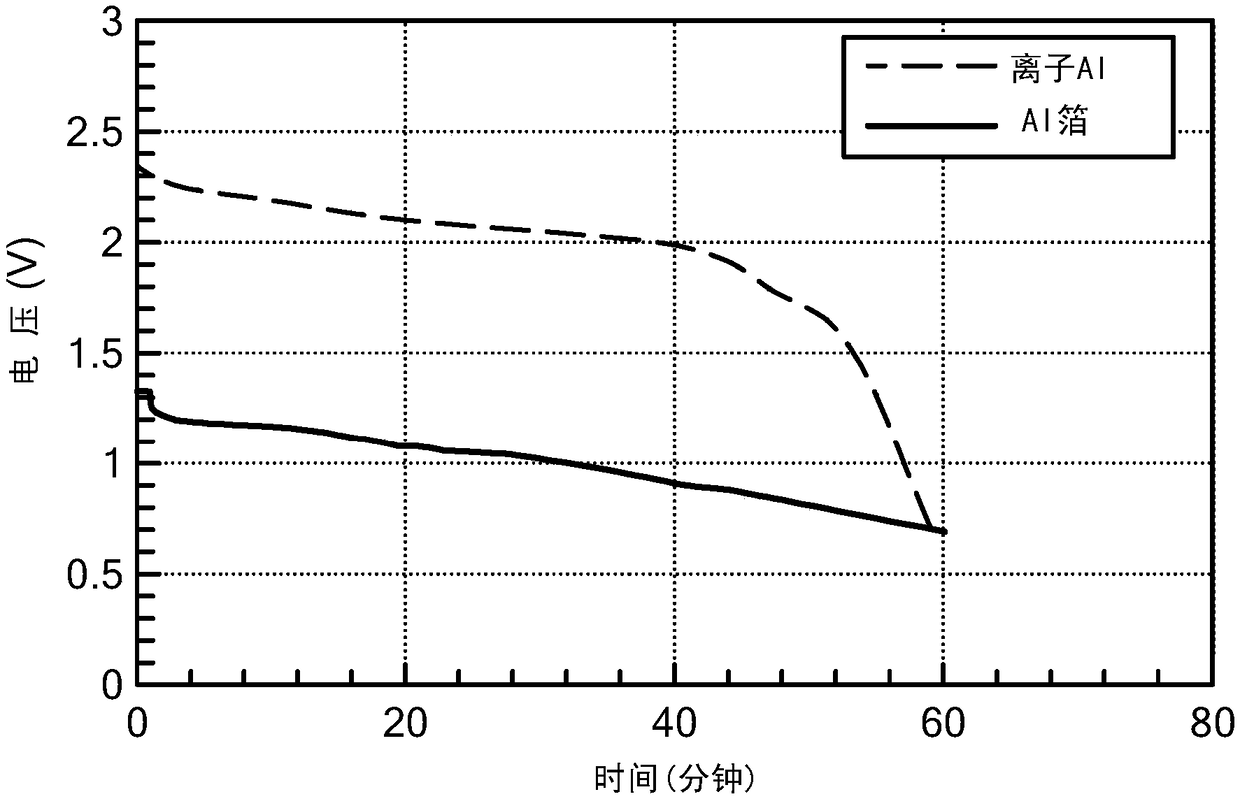
Battery having aluminum anode and solid polymer electrolyte
A conductive polymer and battery technology, applied in the field of electrochemical batteries, can solve problems such as expensive and low system specific volume
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0130] The solid ion-conducting polymer material was synthesized in two aspects to describe how the material can be used in different aspects of battery design:
Embodiment 1A
[0132] The PPS polymer was mixed with the ionic compound LiOH monohydrate in a proportion of about 10-40% (by weight) of the metal hydroxide, respectively, and mixed using jet milling. The undoped mixture was heated at 320-380°C and extruded into a 10-15 micron thick film. The extruded film was then annealed at 150 °C for about 2 hours to increase the crystallinity of the blended PPS polymer. In a tube furnace under vacuum at a temperature of 190-200 °C, chloranil dopant was added to the resulting mixture by steam doping for 1-16 hours to synthesize a solid ion-conducting polymer material (PPS / LiOH / Chloranil). The synthesized material underwent a color change during doping, indicating that a chemical reaction had occurred and indicating the formation of a solid ionically conductive polymer material.
Embodiment 1B
[0134] In one aspect, solid ion in particulate form was synthesized by first mixing the DDQ dopant and the base polymer PPS at a ratio of 1 mole DDQ per 4.2 moles of PPS monomer, and heating between 250°C and 325°C to form a particulate form Conductive polymer material (PPS / LiOH / DDQ). Ionic LiOH is mixed into the specific form to produce the solid ionically conductive polymer material.
[0135] The electronic conductivity of the materials in Examples 1A and 1B was measured using a potentiostatic method between blocking electrodes and was determined to be about 6.5 x 10 -9 S / cm (less than or conductivity less than 1×10 -8 S / cm).
[0136] Ionic diffusivity measurements were performed on the compression-molded pellet form of the PPS / LiOH / DDQ material using basic NMR techniques. Specifically, the diffusivity of lithium and hydroxide ions was evaluated by pulsed gradient spin echo ("PGSE") lithium NMR method. PGSE-NMR measurements were performed using a Varian-S Direct Drive 30...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com