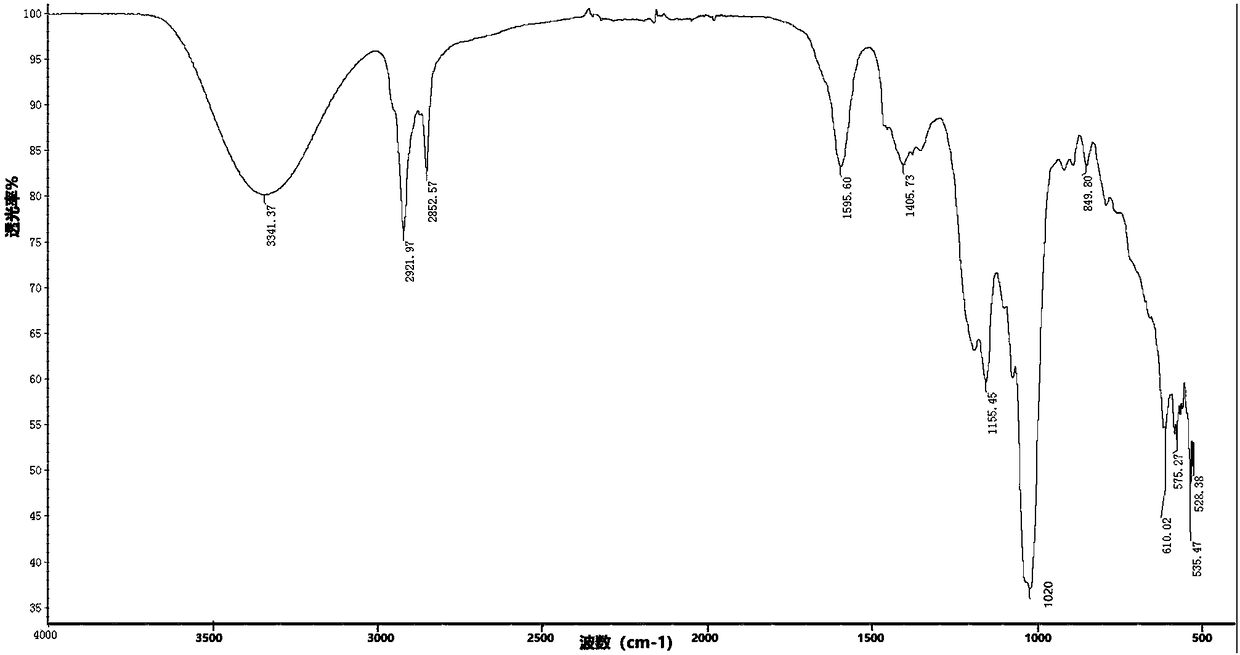
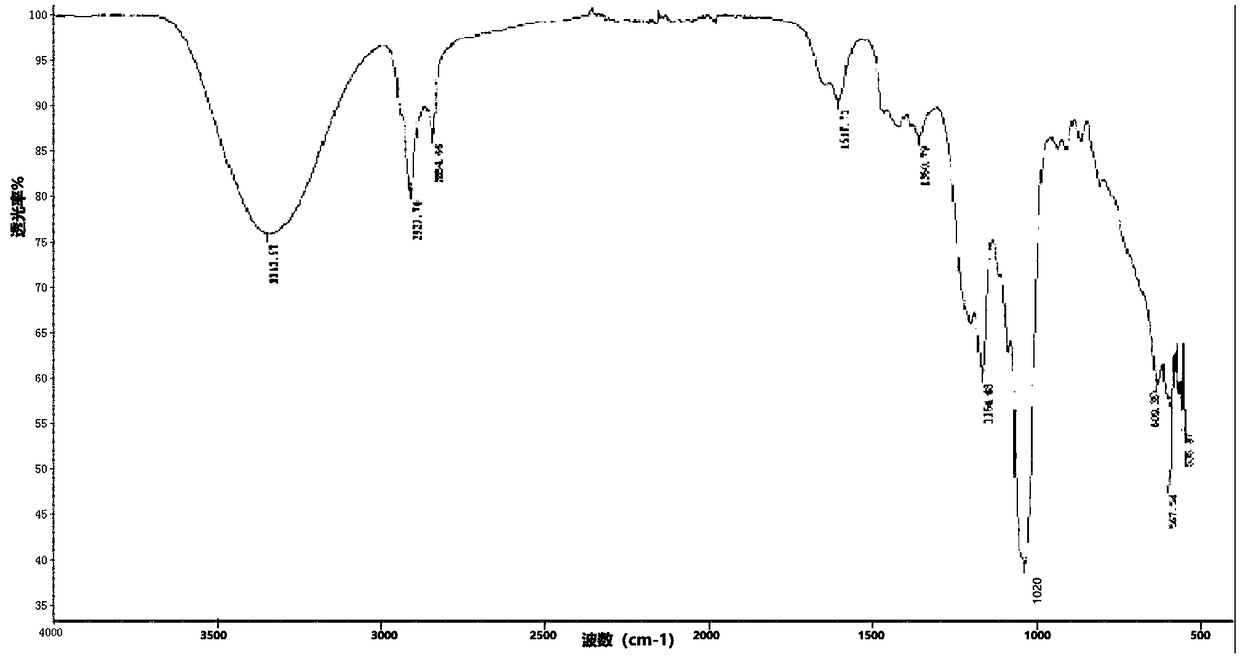
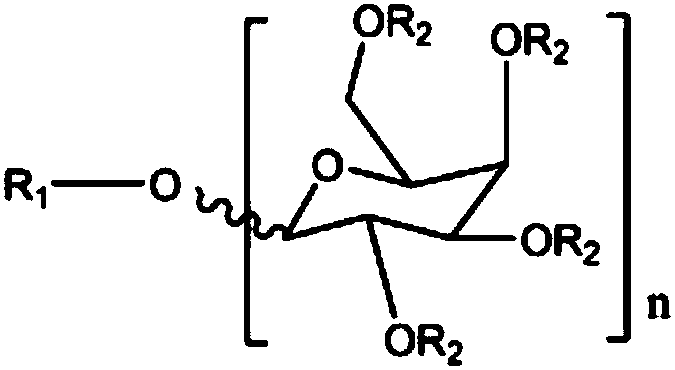
Synthesis process of sodium alkyl polyglucoside hydroxypropyl sulfonate
The technology of alkyl polyglycoside sodium hydroxypropyl sulfonate and alkyl polyglycoside, which is applied in the field of surfactant preparation, can solve the problems of complex operation, undisclosed process, and undisclosed removal of solvent alcohol, etc., and achieves simple preparation process, Solve the effect of poor water solubility and improve water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 73g (0.45mol) of Na 3 PO 4 1. Add 110mL of water into a 250mL four-neck flask at one time to make a 40% aqueous solution, control the temperature at 50°C, add 88.5g (0.45mol) of 3-chloro-2-hydroxypropanesulfonate solid under stirring, and heat up to 55°C After reacting for 4 hours, it was cooled to 10° C., kept for 5 hours to crystallize, and 89 g of solid matter was removed by filtration to obtain 180 g of an aqueous solution of sodium 2,3-epoxypropanesulfonate, with a concentration of 50% and a salt rejection rate of 98%.
Embodiment 2
[0026] In a 500mL four-necked flask equipped with a stirrer, a thermometer, and a condenser tube, add a commercially available 50% solid content of alkyl polyglycoside (APG0810, average degree of polymerization n=1.4) aqueous solution 100g (0.135mol), and implement 43.2g (0.135mol) of the 50% aqueous solution of the intermediate 2,3-epoxypropanesulfonate sodium in Example 1, and 0.72g of solid NaOH catalyst, stirred and heated to 90°C, and after 5 hours of reaction, a transparent light yellow Alkyl polyglycoside sodium hydroxypropyl sulfonate aqueous solution (APG0810, average degree of polymerization n=1.4, 1:1 type).
Embodiment 3
[0028] In a 500mL four-neck flask equipped with a stirrer, a thermometer, and a condenser tube, add a commercially available 50% solid content of alkyl polyglycoside (APG1214, average degree of polymerization n=1.4) aqueous solution 100g (0.115mol), and implement 36.8g (0.115mol) of the 50% aqueous solution of the intermediate 2,3-epoxypropanesulfonate sodium in Example 1, and 0.69g of solid NaOH catalyst, stirred and heated to 100°C, and after 6 hours of reaction, a transparent light yellow Alkyl polyglycoside sodium hydroxypropyl sulfonate aqueous solution (APG1214, average degree of polymerization n=1.4, 1:1 type).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com