Preparation method and application of graphene oxide anti-tumor drug targeting vector
An anti-tumor drug and graphene technology, applied in anti-tumor drugs, drug delivery, drug combination, etc., can solve the problems of inability to reach the dose of lethal cancer cells, limited drug loading, and no clinical application value, etc., and achieve easy modification , large surface area and high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
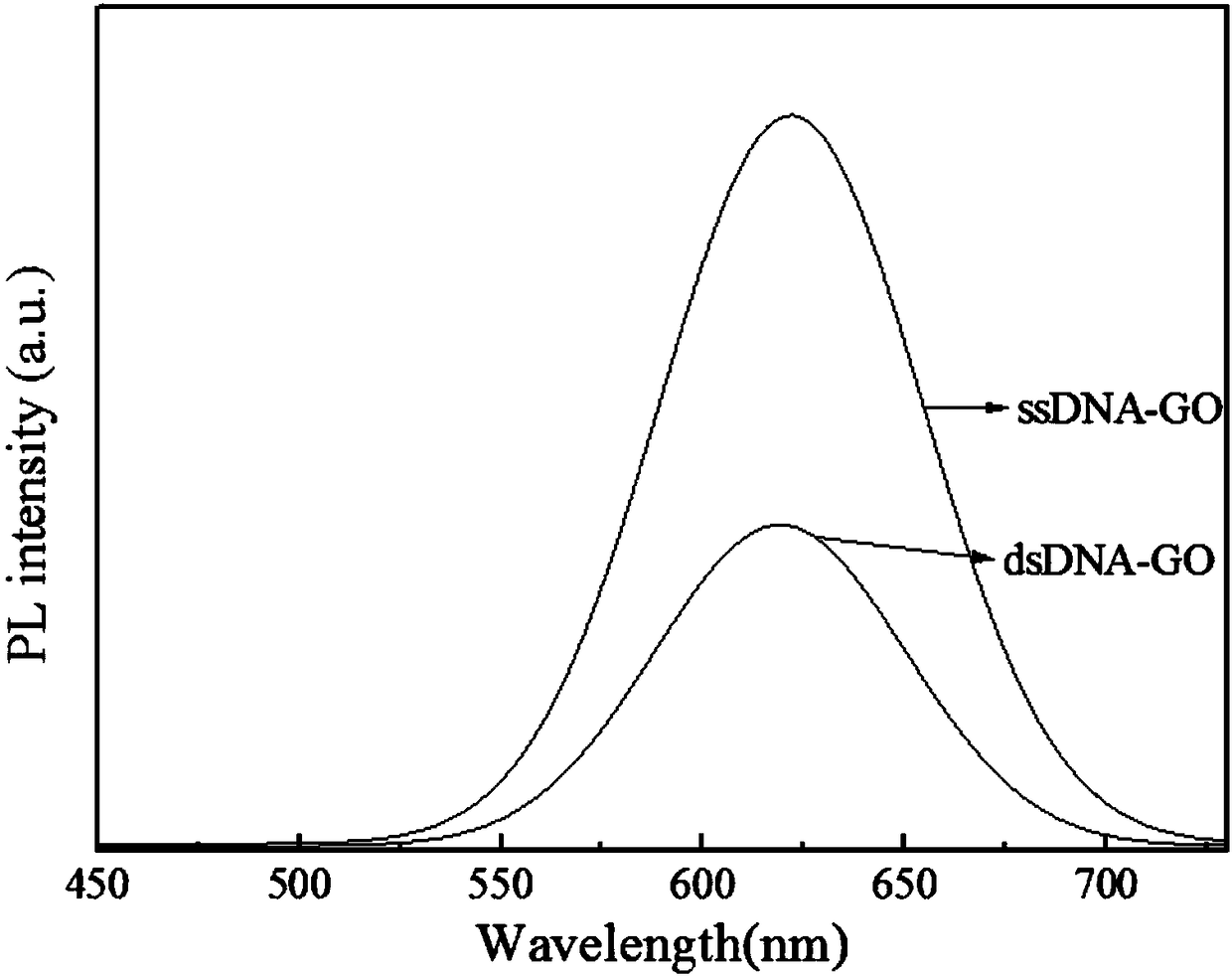
Image
Examples
preparation example Construction
[0029] A preparation method for a graphene oxide antitumor drug targeting carrier, comprising the steps of:
[0030] (1) Dissolving the DNA single strand in PBS buffer solution, ultrasonically dissolving, and then adding the complementary strand of the upper DNA single strand to the above-mentioned cooled solution to perform base complementary pairing of double-stranded DNA molecules;
[0031] (2) Graphene oxide is added in the PBS buffer solution, ultrasonically dissolved, then the DNA solution of complementary base pairing in step (1) is added in the solution graphene oxide solution, stirring and mixing;
[0032] (3) Add the antitumor drug to the mixed solution in step (2), stir and mix evenly, and centrifuge to obtain a precipitate that is the graphene oxide antitumor drug targeting carrier.
[0033] Preferably, in the steps (1) and (2), the concentration of the PBS buffer solution is 0.01 mol / L, and the pH is 7.0-8.0.
[0034] Preferably, in the step (1), in a thermostat,...
Embodiment 1
[0041] 0.01M phosphate buffer: weigh 8g NaCl, 0.2g KCl, 1.44g Na 2 HPO 4 and 0.24g KH 2 PO 4 , dissolved in 800ml of distilled water, adjusted the pH value of the solution to 7.4 with HCl, and finally added distilled water to make the volume to 1L. Steam sterilize at 15lbf / in2 (1034×105Pa) under high pressure (at least 20 minutes), and store at room temperature or in a refrigerator at 4°C.
[0042] (1) Dissolve 10 μL ssDNA (10 μM) in 0.01M PBS buffer solution, ultrasonically dissolve, heat to 95°C in a thermostat and keep it for 10 minutes, then naturally cool to 25°C;
[0043] Then add 10 μL, 10 μM) complementary strands of ssDNA into the above-mentioned cooled solution for base complementary pairing, the temperature of base complementary pairing is 25°C, and the reaction time is 24 hours, to obtain double-stranded DNA molecules;
[0044] (2) 0.2g graphene oxide is added in 0.01M PBS buffer solution, sonication is dissolved, and then the DNA solution of complementary base...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com