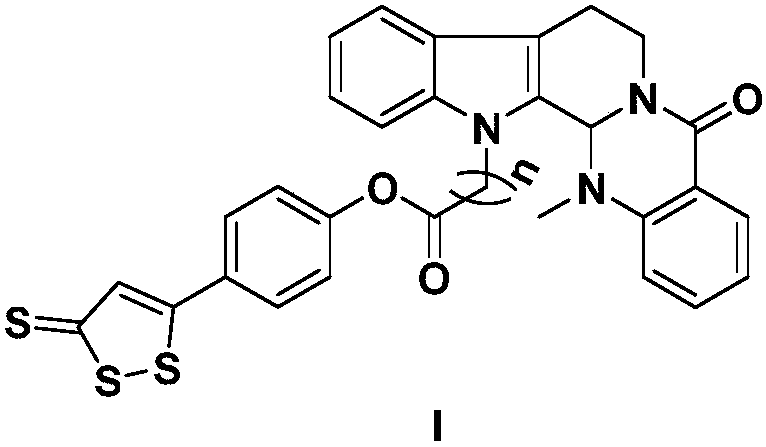
Compound of ADT-OH type H2S donor and evodiamine and preparation method thereof and application
A technology of ADT-OH and evodiamine, which is applied in the field of natural medicine and medicinal chemistry, can solve the problems of uncontrolled release, high toxicity, and inability to be directly applied to clinical research.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
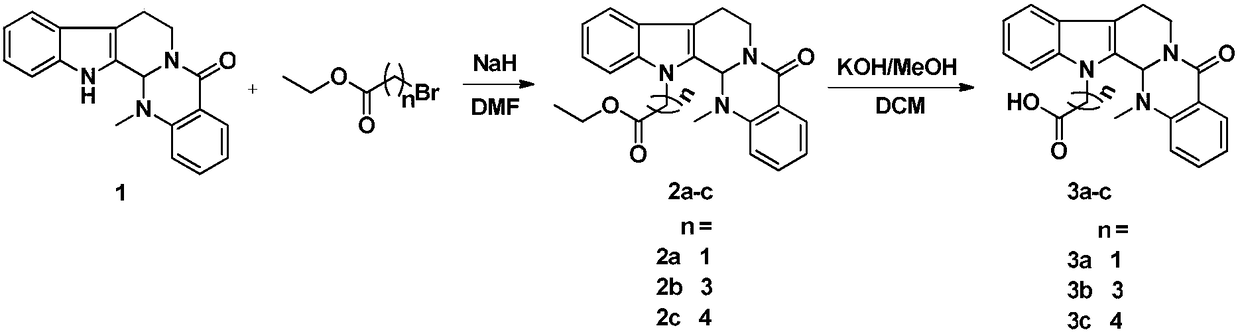
Examples
Embodiment 1
[0021]
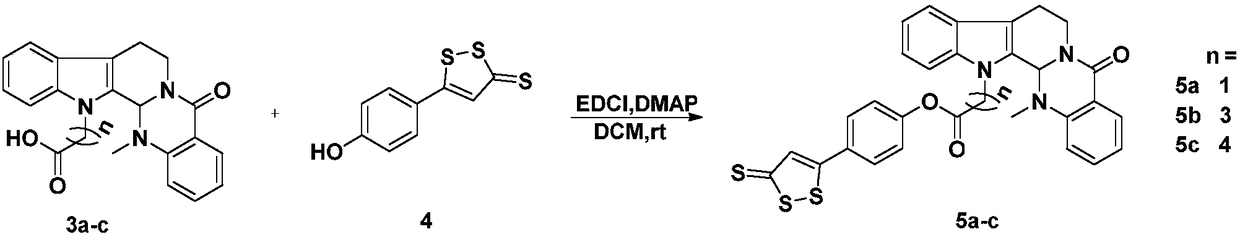
[0022] Take evodiamine intermediate 3a (20mg, 0.05mmol), dissolve it in dichloromethane (5mL), and add nortrisulfide (4, 20mg, 0.08mmol), EDCI (45mg, 0.25mmol), DMAP (4mg , 0.02mmol), the reaction was stirred at room temperature, the progress of the reaction was monitored by TCL, and the reaction was terminated after 12h. The reaction solution was poured into 10 mL ice-water mixture, extracted with dichloromethane (10 mL×3), washed with saturated saline solution, dried over anhydrous sodium sulfate, recovered dichloromethane, and obtained crude product 5a, which was passed through a silica gel column (petroleum ether: acetic acid Ethyl ester=8:1), separated to obtain a brown-red solid with a yield of 70%. MS(ESI)m / z calcd for C 30 h 23 N 3 HO 3 S 3 [M+H] + 569.1, found 569.8. 1 H NMR (CDCl 3 ,400MHz)δ8.15(d,J=7.8Hz,1H,Ar-H),7.66(m,3H,Ar-H),7.52(m,2H,Ar-H),7.36(m,3H,Ar-H) -H),7.30(m,1H,Ar-H),7.25(m,1H,Ar-H),7.23(s,1H,4H-1,2-dithiole-3-thione),7.17(d,J =7.8...
Embodiment 2
[0024]
[0025] Compound 5b was prepared according to the synthesis method of Example 1. Brown-red solid, yield 30%. HRMS(ESI)m / zcalcd for C 32 h 27 N 3 NaO 3 S 3 [M+Na] + 620.1040, found 620.1107. 1 H NMR (CDCl 3 ,400MHz)δ8.14(dd,J=7.8,1.2Hz,1H,Ar-H),7.72(m,1H,Ar-H),7.61(d,J=8.5Hz,2H,Ar-H), 7.53(m,1H,Ar-H),7.50(m,1H,Ar-H),7.46(m,1H,Ar-H),7.38(s,1H,4H-1,2-dithiole-3-thione ),7.31(m,1H,Ar-H),7.21(m,2H,Ar-H),7.06(d,J=8.5Hz,2H,Ar-H),6.01(s,1H,N-CH- N),4.92(m,1H,N-CH 2 ),4.57(m,1H,13-N-CH 2 ),4.36(m,1H,13-N-CH 2 ),3.20(m,1H,N-CH 2 ),3.04(m,1H,-CH 2 ),2.91(m,1H,-CH 2 ),2.65(m,2H,-CH 2 ),2.41(s,3H,N-CH 3 ),2.30(m,2H,-CH 2 ). 13 C NMR (CDCl 3 ,100MHz)δ215.64,171.61,170.79,164.67,153.38,150.95,137.41,136.18,133.10,131.04,129.41,129.17,128.97,128.32(×2),125.96,124.50,123.30,123.11,122.82(×2),120.02 ,119.31,113.85,109.82,68.21,65.70,50.97,42.90,31.60,25.14,20.49.
Embodiment 3
[0027]
[0028]Compound 5c was prepared according to the synthesis method of Example 1. Brown-red solid, yield 46%. HRMS(ESI)m / zcalcd for C 33 h 29 N 3 NaO 3 S 3 [M+Na] + 634.1263, found 634.1219. 1 H NMR (CDCl 3 ,400MHz)δ8.14(dd,J=7.8,1.2Hz,1H,Ar-H),7.64(d,J=8.7Hz,2H,Ar-H),7.61(m,1H,Ar-H), 7.49(m,1H,Ar-H),7.40(m,1H,Ar-H),7.38(s,1H,4H-1,2-dithiole-3-thione),7.29(m,1H,Ar-H ),7.17–7.24(m,3H,Ar-H),7.13(d,J=8.7Hz,2H,Ar-H),5.99(s,1H,N-CH-N),4.91(m,1H, N-CH 2 ),4.45(m,1H,13-N-CH 2 ),4.28(m,1H,13-N-CH 2 ),3.20(m,1H,N-CH 2 ),3.03(m,1H,-CH 2 ),2.90(m,1H,-CH 2 ),2.62(m,2H,-CH 2 ),2.41(s,3H,N-CH 3 ),1.98(m,2H,-CH 2 ),1.82(m,2H,-CH 2 ). 13 C NMR (CDCl 3 ,100MHz)δ215.60,171.64,171.07,164.65,153.53,150.99,137.25,136.15,133.08,129.35,129.12,128.37,128.32(×2),125.92,124.46,124.25,123.25,122.89(×3),119.84,119.27 ,113.49,109.78,68.24,43.63,39.43,36.63,33.94,29.64,22.46,20.48.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com