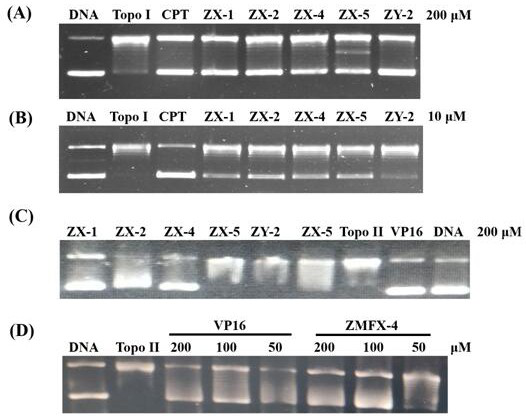
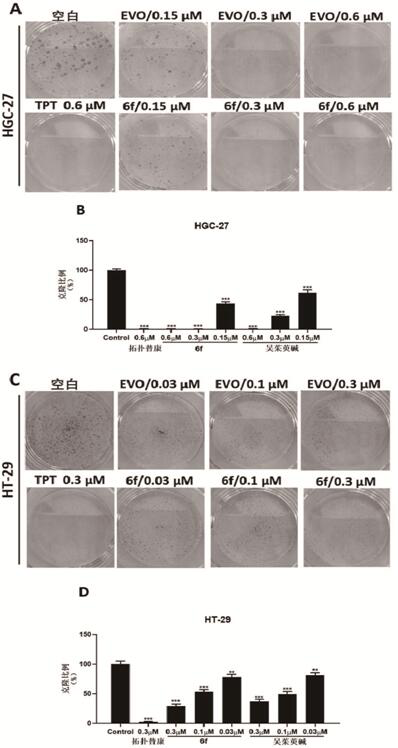
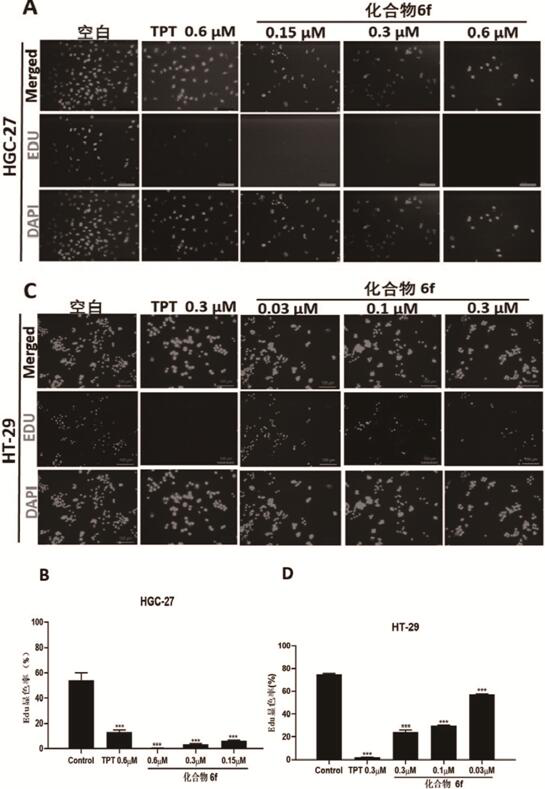
Evodiamine derivative as well as preparation and application thereof
A technology of evodiamine and its derivatives, which is applied in the field of medicine and can solve the problems of reducing the effect of evodiamine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] 10-methoxy-7,8,13,13b-tetrahydro-5H-benzo [5',6'] [1,3] thiazino[3',2':1,2] pyrido [3,4-b]indol -5-one (compound 6a)
[0045] The structural formula of compound 6a is:
[0046] (1) At room temperature, mix 2-(phenylamino)benzoic acid (compound 1-1, 1 mmol), tryptamine (1.2 mmol), 1-ethyl-(3-dimethylaminopropyl) carbonyl di Imine hydrochloride (1.2 mmol), triethylamine (3 mmol), 1-hydroxybenzotriazole (1.3 mmol), dissolved in 10 ml of dichloromethane, reacted for 5 hours, separated by extraction column chromatography to obtain the product N-(2-(1H-indol-3-yl)ethyl)-2-(phenylamino)benzamide (compound 1-2), the yield was 78%;
[0047] The structural formula of compound 1-1 is:
[0048] The structural formula of compound 1-2 is:
[0049] (2) N-(2-(1H-indol-3-yl)ethyl)-2-(phenylamino)benzamide (compound 1-2, 1 mmol), triethyl orthoformate (3 mmol) and boron trifluoride Diethyl ether (0.5 mmol) was added into a 25 mL round bottom flask, and N,N-dimethylformamide wa...
Embodiment 2
[0051] 14-(2-fluorophenyl)-8,13,13b,14-tetrahydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one (compound 6b)
[0052] The structural formula of compound 6b is:
[0053] Replace the raw material 2-(phenylamino)benzoic acid (compound 1-1) in step (1) of Example 1 with 2-((2-fluorophenyl)amino)benzoic acid (compound 2-1), and the rest of the steps are the same as in the example 1 prepared with a yield of 77%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 11.12 (s, 1H), 7.85 (dd, J = 7.8, 1.6 Hz,1H), 7.49 – 7.35 (m, 3H), 7.31 (dt, J = 8.1, 0.9 Hz, 1H), 7.28 – 7.21 (m,2H), 7.19 – 7.14 (m, 1H), 7.04 (m, 2H), 6.95 (ddd, J = 7.9, 7.0, 1.0 Hz, 1H),6.87 (d, J = 8.2 Hz, 1H), 6.48 (s, 1H), 4.62 (dd, J = 13.0, 5.6 Hz, 1H), 3.36(m, 1H), 3.00 – 2.83 (m, 1H), 2.68 (dd, J = 15.5, 4.7 Hz, 1H). 13 C NMR (101MHz, DMSO) δ 165.03, 158.08 (d, J = 248.1 Hz), 145.62, 136.45, 133.68, 133.44(d, J = 10.8 Hz), 131.44, 129.52, 128.33, 128.14 (d, J = 8.0 Hz), 126.60,125.27 (d, J = 3.7 Hz), 1...
Embodiment 3
[0056] 14-(2-chlorophenyl)-8,13,13b,14-tetrahydroindolo[2',3':3,4]pyrido[2,1-b]quinazolin-5(7H)-one (compound 6c)
[0057] The structural formula of compound 6c is:
[0058] Replace the raw material 2-(phenylamino)benzoic acid (compound 1-1) in step (1) of Example 1 with 2-((2-chlorophenyl)amino)benzoic acid (compound 3-1), and the rest of the steps are the same as in the example 1 prepared in a yield of 74%. 1 H NMR (400 MHz, DMSO- d 6 ) δ 10.92 (s, 1H), 7.86 (d, J = 7.8 Hz, 1H),7.56 – 7.22 (m, 7H), 7.00 (m, 3H), 6.67 (d, J = 8.2 Hz, 1H), 6.45 (s, 1H), 4.63 (dd, J = 13.0, 5.7 Hz, 1H), 3.32 (s, 1H), 3.03 – 2.92 (m, 1H), 2.70 (dd, J = 16.1, 4.9 Hz, 1H). 13 C NMR (101 MHz, DMSO) δ 165.12, 145.99, 142.56,136.46, 133.76, 132.30, 131.72, 131.09, 128.89, 128.50, 126.57, 122.19,119.26, 118.55, 112.21, 112.12, 71.06, 42.45, 19.71.
[0059] The structural formula of compound 3-1 is:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com