4-(substituted phenyl)-6-ferrocenyl-3,4-dihydropyrimidine-2(1H)-one and preparation method thereof
A technology of ferrocenyl and dihydropyrimidine, which is applied in the field of chemical synthesis and can solve the problem that substituents have no obvious influence on compound synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Add 0.2281g (1mmol) acetyl ferrocene, 0.0721g (1.2mmol) urea, 0.1273g (1.2mmol) benzaldehyde, 0.0200g (0.74mmol) boric acid and 10mL acetic acid to a three-necked flask, and react at 100°C to The starting material reacted completely (reaction monitored by TLC) for 1 h.
[0042] 2) Pour the reaction mixture into water to precipitate solids, suction filter, wash with water, dry naturally, and recrystallize through absolute ethanol to obtain 4-phenyl-6-ferrocenyl-3,4-dihydropyrimidine- 2(1H)-one.
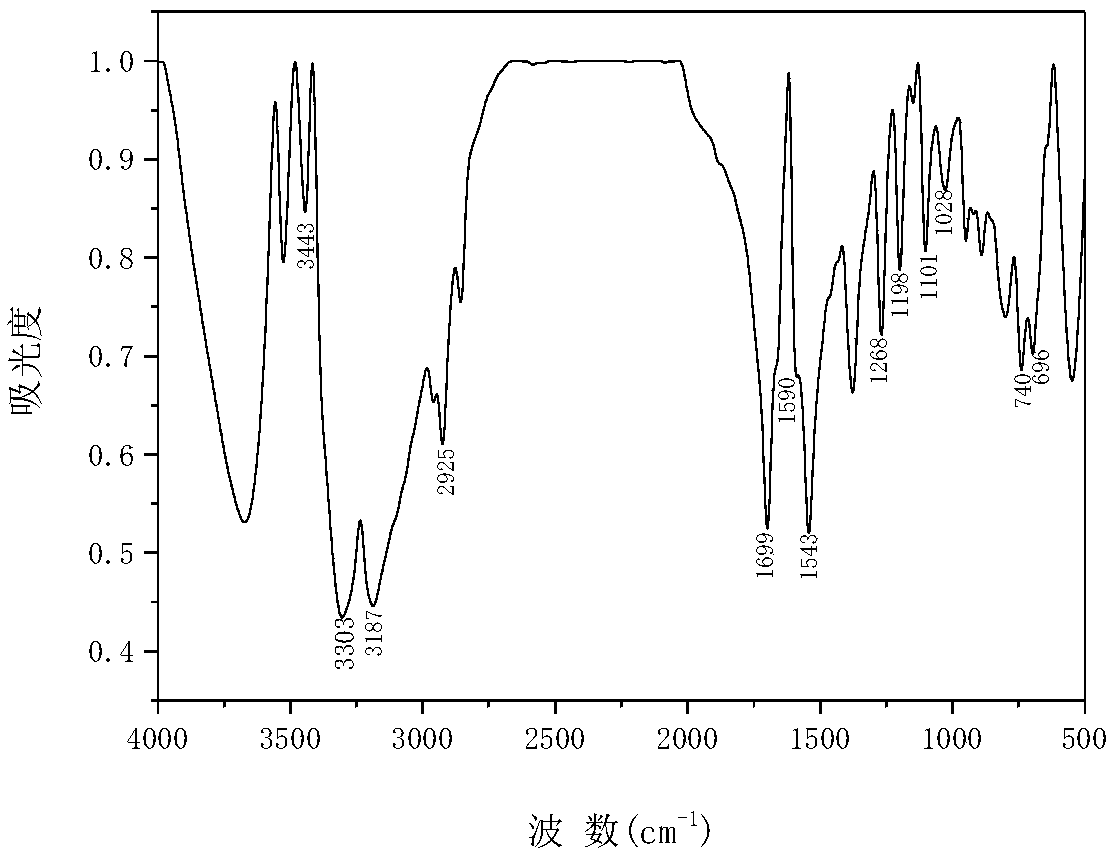
[0043] Yield: 86%; m.p.: 173-175℃; IR (KBr, v / cm -1 ):3443,3303υ(N-H),3187υ(C=C-H),2925υ(-CH-),1699υ(-C=O),1590,1543υ(-C=C,Ar),1268υ(C-N),1198υ( C-C),1101υ as (C-C,Fc),1028γ(C-H,Fc),746,696γ(C-H,Ar-R);
[0044] 1 H NMR (400MHz, CDCl3) δ9.37(s,1H,N-H),7.59(d,1H,Ar-H),7.41(m,1.5Hz,2H,Ar-H),7.29(d,2H,Ar-H) -H), 6.86(d, 1H, C=C-H), 6.12(s, 1H, -CH-), 5.63-4.86(m, 4H, Fc-H).
Embodiment 2
[0046] 1) Add 0.2281g (1mmol) acetyl ferrocene, 0.0721g (1.2mmol) urea, 0.1442g (1.2mmol) p-tolualdehyde, 0.0200g (0.74mmol) boric acid and 10mL acetic acid to the three-necked flask, 100 The reaction was carried out at ℃ until the raw materials were completely reacted (monitored by TLC), and the time was 1 h.
[0047]2) Pour the reaction mixture into water to precipitate a solid, suction filter, wash with water, dry naturally, and recrystallize through absolute ethanol to obtain 4-(4-methylphenyl)-6-ferrocenyl-3, 4-Dihydropyrimidin-2(1H)-one.
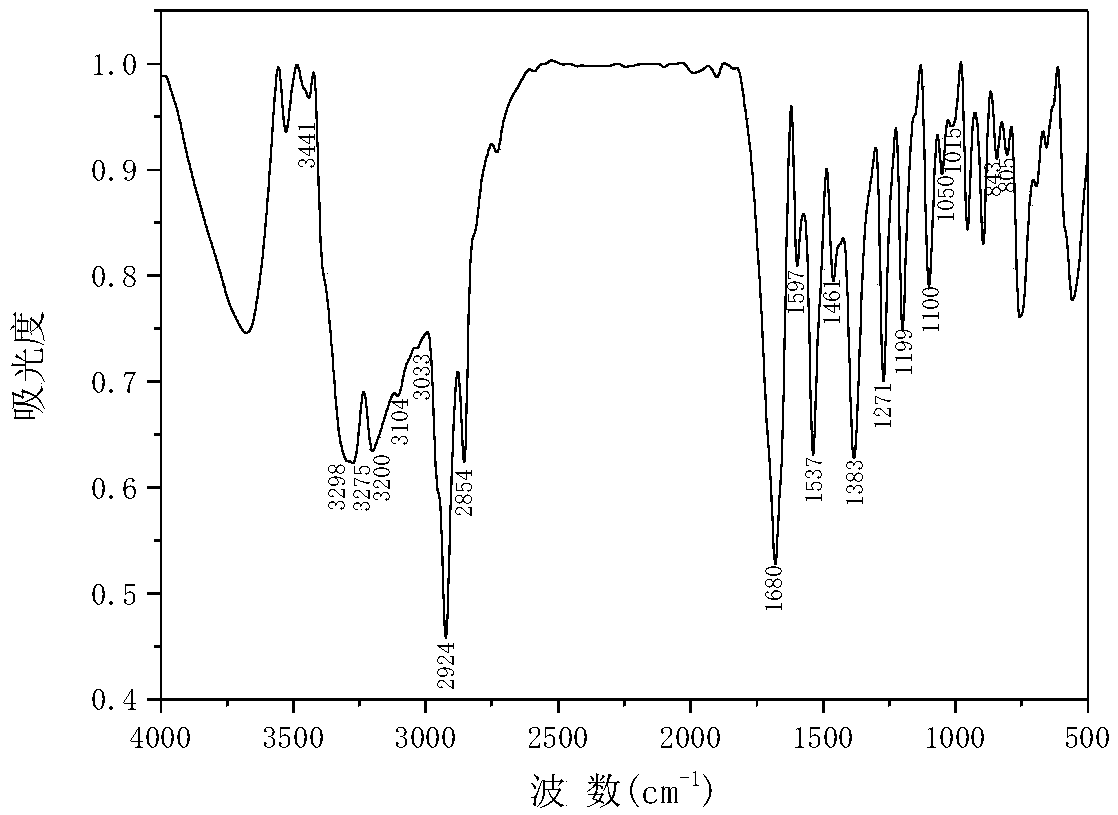
[0048] Yield: 86.3%; m.p.: 133-136℃; IR (KBr, v / cm -1 ):3441,3298,3275(N-H),3200,3104,3033(C=C-H),2924υ(-CH-),2854υ(-CH 3 ), 1680υ(-C=O), 1597,1537,1461υ(-C=C, Ar), 1383δs(-CH 3 ),1271υ(C-N),1199υ(C-C),1100υ as (C-C,Fc),1050,1015γ(C-H,Fc),843,805γ(C-H,Ar-R);
[0049] 1 H NMR (400MHz, CDCl3) δ9.35(s,1H,N-H),7.58(d,1H,Ar-H),7.29(d,2H,Ar-H),7.09(d,1H Ar-H), 6.84(d,1H,C=C-H),6.07(s,1H,-CH-),5.60-5.05(m,4H,Fc-H),2.28(s,3H,-CH 3 ). ...
Embodiment 3
[0051] 1) Add 0.2281g (1mmol) acetyl ferrocene, 0.0721g (1.2mmol) urea, 0.1634g (1.2mmol) p-methoxybenzaldehyde, 0.0200g (0.74mmol) boric acid and 10mL acetic acid to the three-necked flask, The reaction was carried out at 100° C. until the raw materials were completely reacted (reaction monitored by TLC), and the time was 1 h.
[0052] 2) Pour the reaction mixture into water to precipitate solids, filter with suction, wash with water, dry naturally, and recrystallize through absolute ethanol to obtain 4-(4-methoxyphenyl)-6-ferrocenyl-3 , 4-Dihydropyrimidin-2(1H)-one.
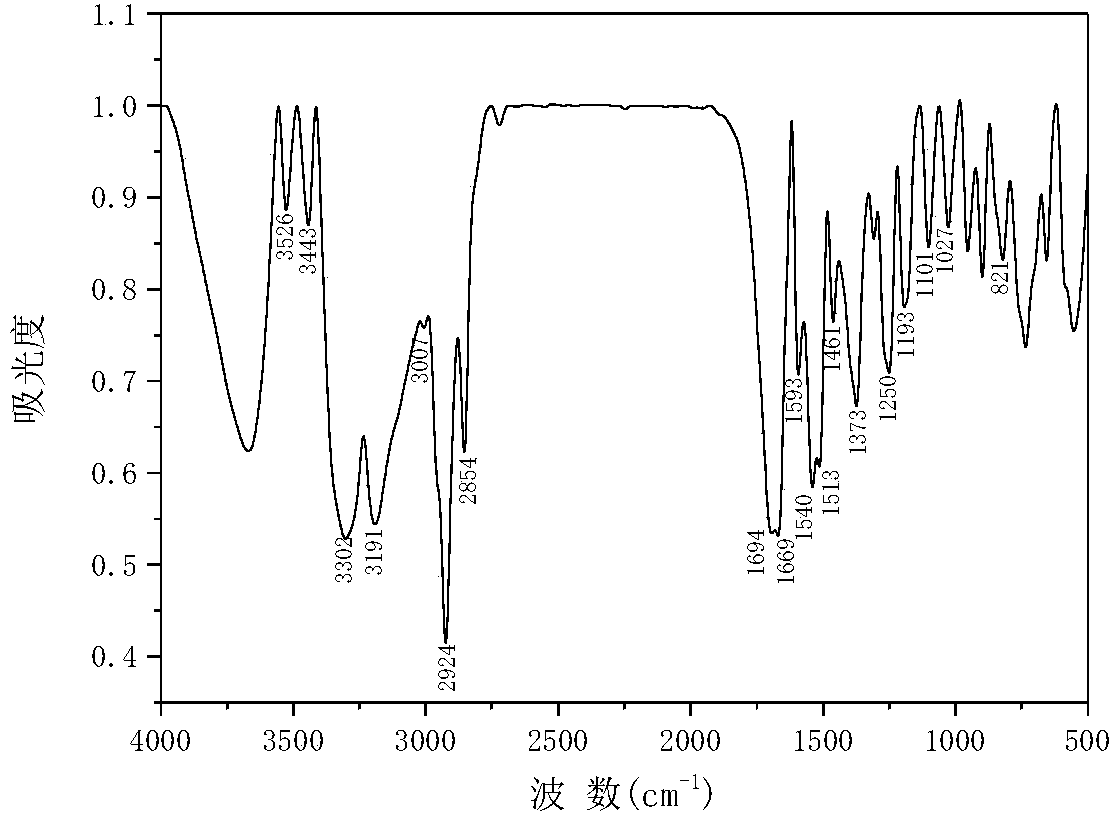
[0053] Yield: 87%; m.p.: 129-131℃; IR (KBr, v / cm -1 ):3443,3302υ(N-H),3191,3007υ(C=C-H),2924υ(-CH-),2854υ(-CH 3 ), 1694υ(-C=O), 1669υ(-C=C), 1593,1540,1513, 1461υ(-C=C, Ar), 1373δs(-CH 3 ),1250υ(C-N),1193υ(C-C),1101υ as (C-C, Fc), 1027γ (C-H, Fc), 821γ (C-H, Ar-R);
[0054] 1 H NMR(400MHz, CDCl3)δ9.35(s,1H,N-H),7.55(d,1H,Ar-H),7.33(d,2H,Ar-H),6.84(d,1H,Ar-H) ,6.80(d,1H,C=C-H),6.06(s,1H,-CH-),5.56-5.17(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com