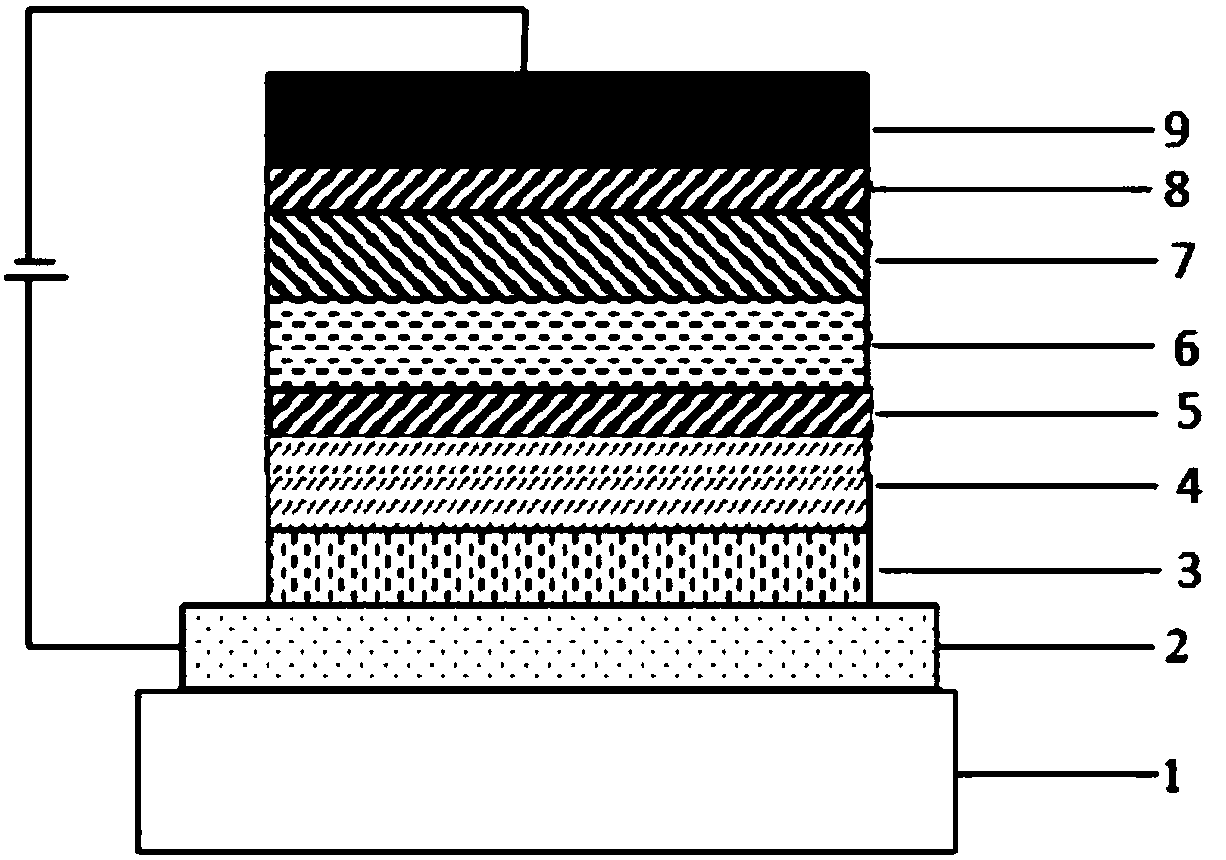
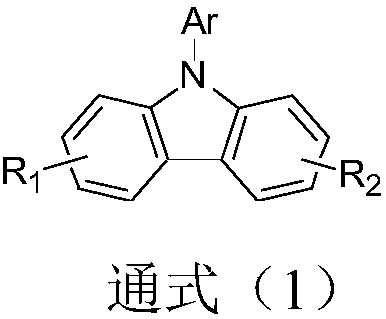
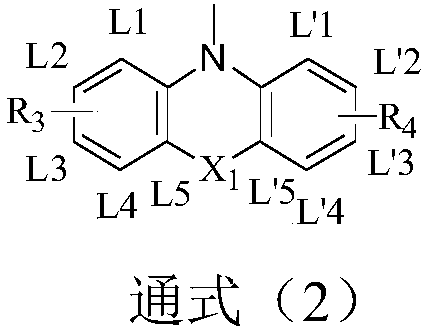
Compound taking carbazole as core, and applications thereof in organic light-emitting devices
A compound and carbazole technology, applied in the application field of organic electroluminescent devices, can solve the problems of different performance, achieve high glass transition temperature, reduce efficiency roll-off, and improve hole injection/transport performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the synthesis of compound 7:
[0045] synthetic route:
[0046]
[0047] In a 250mL three-neck flask, under an atmosphere of nitrogen gas, add 0.02mol 2-bromocarbazole, 0.03mol bromobenzene, 0.04mol sodium tert-butoxide, 10 -4 mol Pd 2 (dba) 3 , 10 -4 mol P(t-Bu) 3 and 200mL of toluene, heated to reflux for 10 hours, sampled and spotted, the raw materials reacted completely; naturally cooled to room temperature (20-25°C), filtered, and the filtrate was collected for vacuum rotary evaporation (-0.09MPa, 85°C), and column chromatography , to obtain the target product raw material A1;
[0048] In a 250mL three-necked flask, under nitrogen protection, add 0.03mol dibenzofuran-2-amine, 0.04mol 2-(3-bromonaphthyl) propan-2-ol, 150mL toluene and stir to mix, then add 0.05mol t- Sodium butoxide, 0.0015mol Pd 2 (dba) 3 , 0.0015mol tri-tert-butylphosphine, heated to 115°C, and refluxed for 24 hours; naturally cooled to room temperature, filtered, and the ...
Embodiment 2
[0053] Embodiment 2: the synthesis of compound 8:
[0054] synthetic route:
[0055]
[0056]
[0057] In a 250mL three-neck flask, under nitrogen protection, add 0.04mol 9,9-dimethyl-9H-fluoren-3-ol, add 100mL acetic acid, stir and dissolve at room temperature, and slowly add 0.05 mol Br 2 50 mL of acetic acid solution, stirred at room temperature for 12 hours. Add NaOH aqueous solution (2mol / L) to neutralize to neutral, solid precipitates out, filter to take filter cake, dry under vacuum, pass through neutral silica gel column to obtain 4-bromo-9,9-dimethyl-9H-fluorene -3-ol;
[0058] In a 250mL three-neck flask, under nitrogen protection, add 0.04mol 4-bromo-9,9-dimethyl-9H-fluoren-3-ol, add 30% ammonia water (volume ratio) 100mL, heat to 80°C for reaction 6 hours. The system was cooled to room temperature, and evaporated under reduced pressure (-0.09MPa, 120°C), and passed through a neutral silica gel column to obtain 4-amino-9,9-dimethyl-9H-fluoren-3-ol;
[00...
Embodiment 3
[0063] Embodiment 3: the synthesis of compound 14:
[0064] synthetic route:
[0065]
[0066] In a 250mL three-neck flask, under nitrogen protection, add 0.04mol 9,9-dimethyl-9H-fluoren-3-ol, add 100mL acetic acid, stir and dissolve at room temperature, and slowly add 0.05 mol Br 2 50mL of acetic acid solution, stirred at room temperature for 12 hours; added NaOH aqueous solution (2mol / L) to neutralize to neutrality, solid precipitated, filtered to take filter cake, dried under vacuum, and passed through a neutral silica gel column to obtain 2-bromo-9 ,9-Dimethyl-9H-fluoren-3-ol;
[0067] In a 250mL three-neck flask, under nitrogen protection, add 0.04mol 2-bromo-9,9-dimethyl-9H-fluoren-3-ol, add 30% ammonia water (volume ratio) 100mL, heat to 80°C for reaction 6 hours; the system was cooled to room temperature, vacuum rotary evaporation (-0.09MPa, 120°C), and passed through a neutral silica gel column to obtain 2-amino-9,9-dimethyl-9H-fluoren-3-ol;
[0068] In a 250mL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com