Liquid-phase synthesis method of palmitoyl tripeptide-1
A technology of palmitoyl tripeptide and liquid phase synthesis, which is applied in the field of drug synthesis, can solve the problems of high cost, non-environmental protection, and complicated process, and achieve the effects of low cost, environmental protection, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
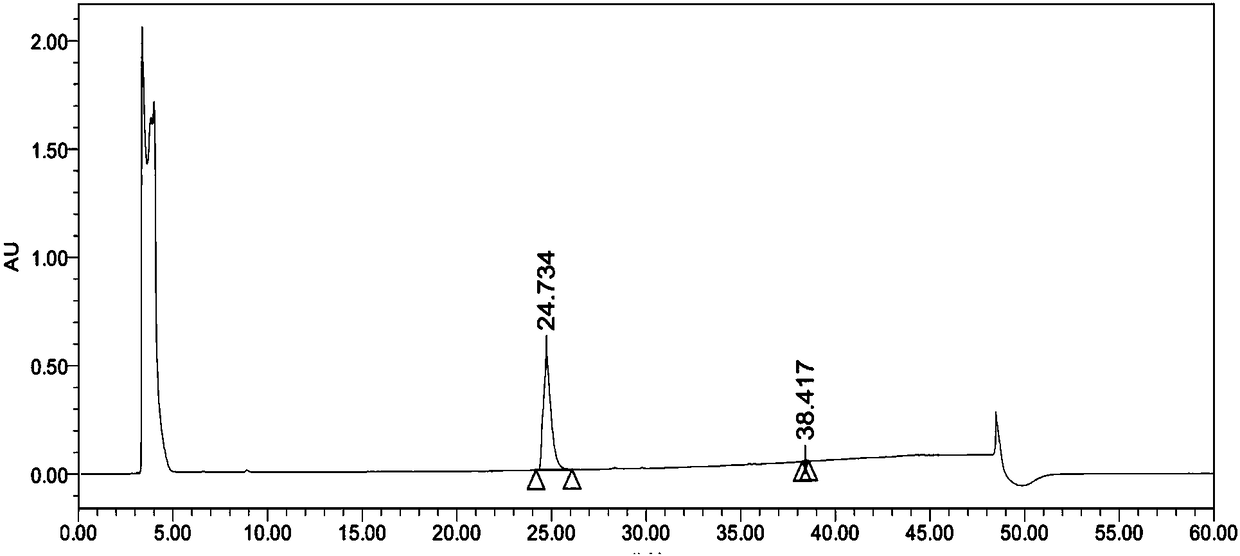
Image
Examples
Embodiment 1
[0027] A kind of embodiment of palmitoyl tripeptide-1 liquid phase synthesis method of the present invention, comprises the following steps:
[0028] (1) Synthesis of Pal-Gly-OH
[0029] According to the molar ratio of glycine, sodium hydroxide and palmitoyl chloride as 1.0:2.2:1.1, put 15.1g of glycine in a 500mL reaction flask, add 75.5mL of tert-butanol, and add 22.5mL of 30% mass fraction under stirring conditions sodium hydroxide solution, stirred until clear; cooled to 0-5 ° C, and at the same time 67.2 mL of palmitoyl chloride and 22.5 mL of sodium hydroxide solution with a mass fraction of 30% were added dropwise, the temperature was controlled below 5 ° C, and the pH of the reaction system was controlled. At 8.5, after the dropwise addition, warm up to room temperature and stir for 2 hours, cool down to below 10°C, add hydrochloric acid dropwise to adjust the pH to 2-3, filter, wash with water, and dry to obtain the crude product, which is refluxed with 300mL dichloro...
Embodiment 2
[0042] An embodiment of the palmitoyl tripeptide-1 liquid phase synthesis method of the present invention, except that the molar weight of palmitoyl chloride used in the step (1) of the synthesis of Pal-Gly-OH is 1.3 of the molar weight of palmitoyl chloride in embodiment 1 Except that, other steps were the same as in Example 1 to obtain 53.2 g of palmitoyl tripeptide-1, with a yield of 92.3% and a purity greater than 98%.
Embodiment 3
[0044] An embodiment of the palmitoyl tripeptide-1 liquid phase synthesis method of the present invention, except that the molar weight of palmitoyl chloride used in the step (1) of the synthesis of Pal-Gly-OH is 9% of the molar weight of palmitoyl chloride in embodiment 1 / 10 times, other steps are the same as in Example 1, the quality of palmitoyl tripeptide-1 obtained is 51.8g, the yield is 90%, and the purity is greater than 98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com