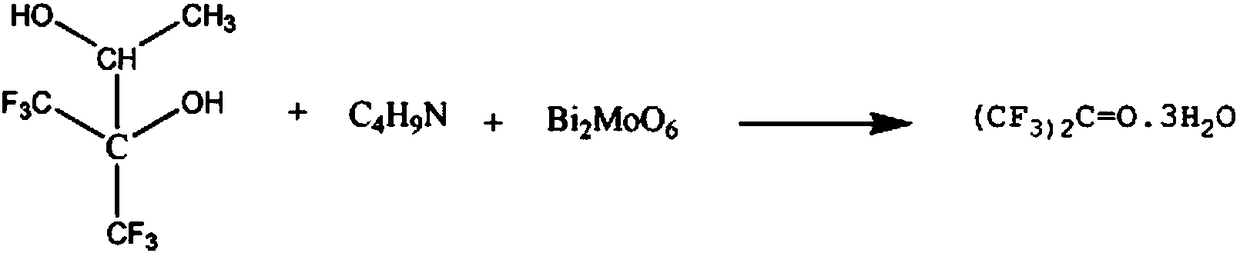Pharmaceutical raw material hexafluoroacetone synthesis method
A technology of hexafluoroacetone and synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve problems such as complicated process and low final yield, and achieve reduction of intermediate links and shortening of reaction Time, the effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0010] Add 3mol of 2,3-dihydroxy-hexafluoroisopentane and 4mol of 60% N-methylpropionamide solution into the reaction vessel, increase the temperature of the solution to 70°C, keep it for 60min, and then add 2mol of Bismuth molybdate, continue to react for 50min, lower the temperature to 40°C, and distill under reduced pressure at 10kPa, collect the fraction at 80°C, wash with 70% m-cresol solution, and wash with 80% m-chloroaniline The solution was washed, and the methoxytoluene solution with a mass fraction of 92% was recrystallized to obtain 478.08 g of crystalline hexafluoroacetone, with a yield of 96%.
example 2
[0012] Add 3 mol of 2,3-dihydroxy-hexafluoroisopentane and 5 mol of N-methylpropionamide solution with a mass fraction of 65% in the reaction vessel, raise the temperature of the solution to 75°C, keep it for 70min, and then add 2.5 mol Bismuth molybdate, continue to react for 60min, reduce temperature to 45°C, 15kPa vacuum distillation, collect 85°C cuts, mass fraction is 73% m-cresol solution washing, mass fraction is 82% m-chloroaniline solution washing, The methoxytoluene solution with a mass fraction of 93% was recrystallized to obtain 483.06 g of crystalline hexafluoroacetone, with a yield of 97%.
example 3
[0014] Add 3mol of 2,3-dihydroxy-hexafluoroisopentane and 6mol of 68% N-methylpropionamide solution into the reaction vessel, increase the temperature of the solution to 80°C, keep it for 80min, and then add 3mol of Bismuth molybdate, continue to react for 70min, lower the temperature to 50°C, distill under reduced pressure at 20kPa, collect the cut at 89°C, wash with 76% m-cresol solution, wash with 85% m-chloroaniline solution, mass fraction The methoxytoluene solution with a fraction of 96% was recrystallized to obtain 490.53 g of crystalline hexafluoroacetone with a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com