Non-cell-derived polypeptide-sensitized DC-CIK cells, and construction method and application thereof
A technology of DC-CIK and construction method, which is applied in the direction of animal cells, vertebrate cells, cell culture active agents, etc., and can solve the problems of unclear specific antigen and limited anti-tumor effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
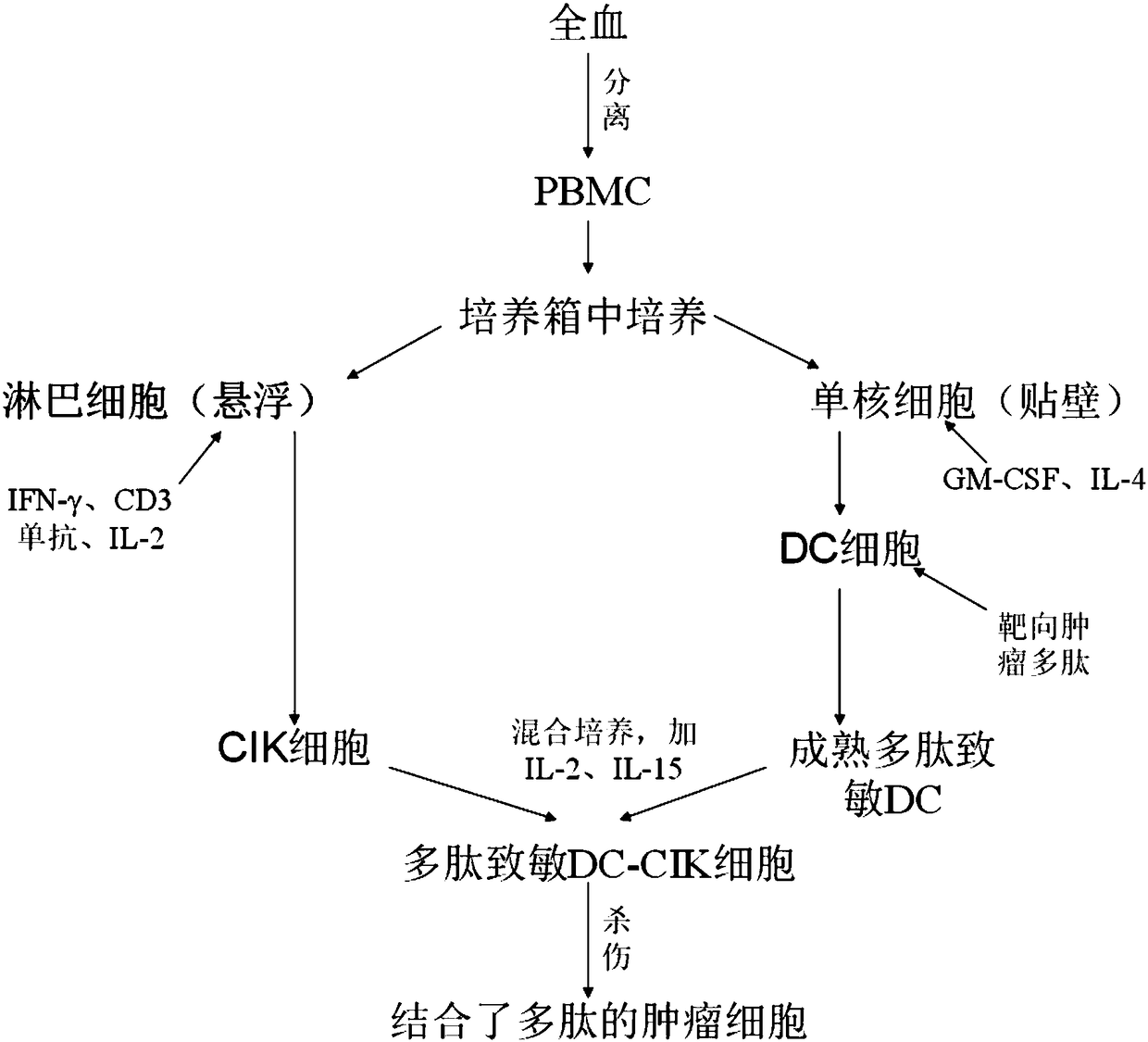
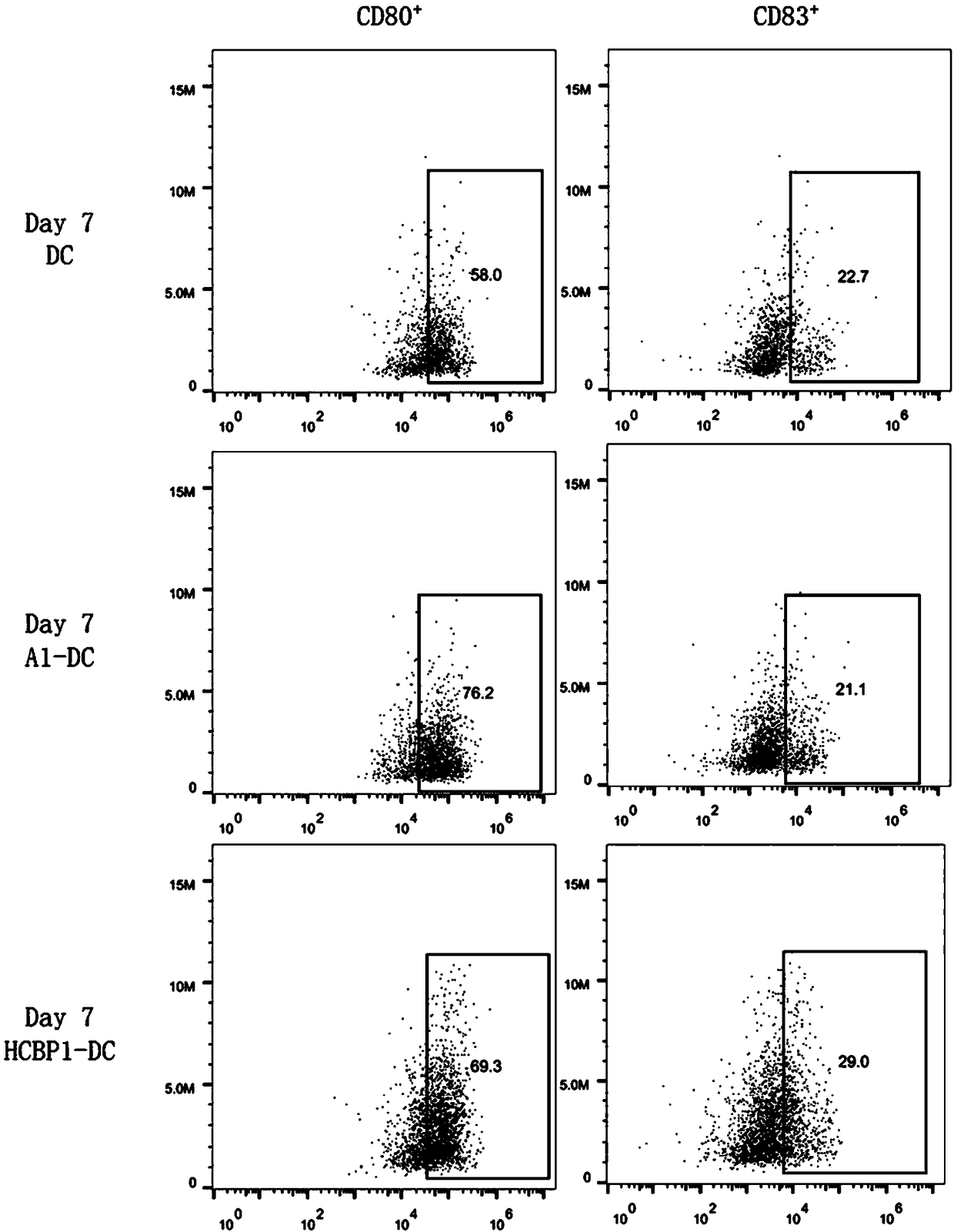
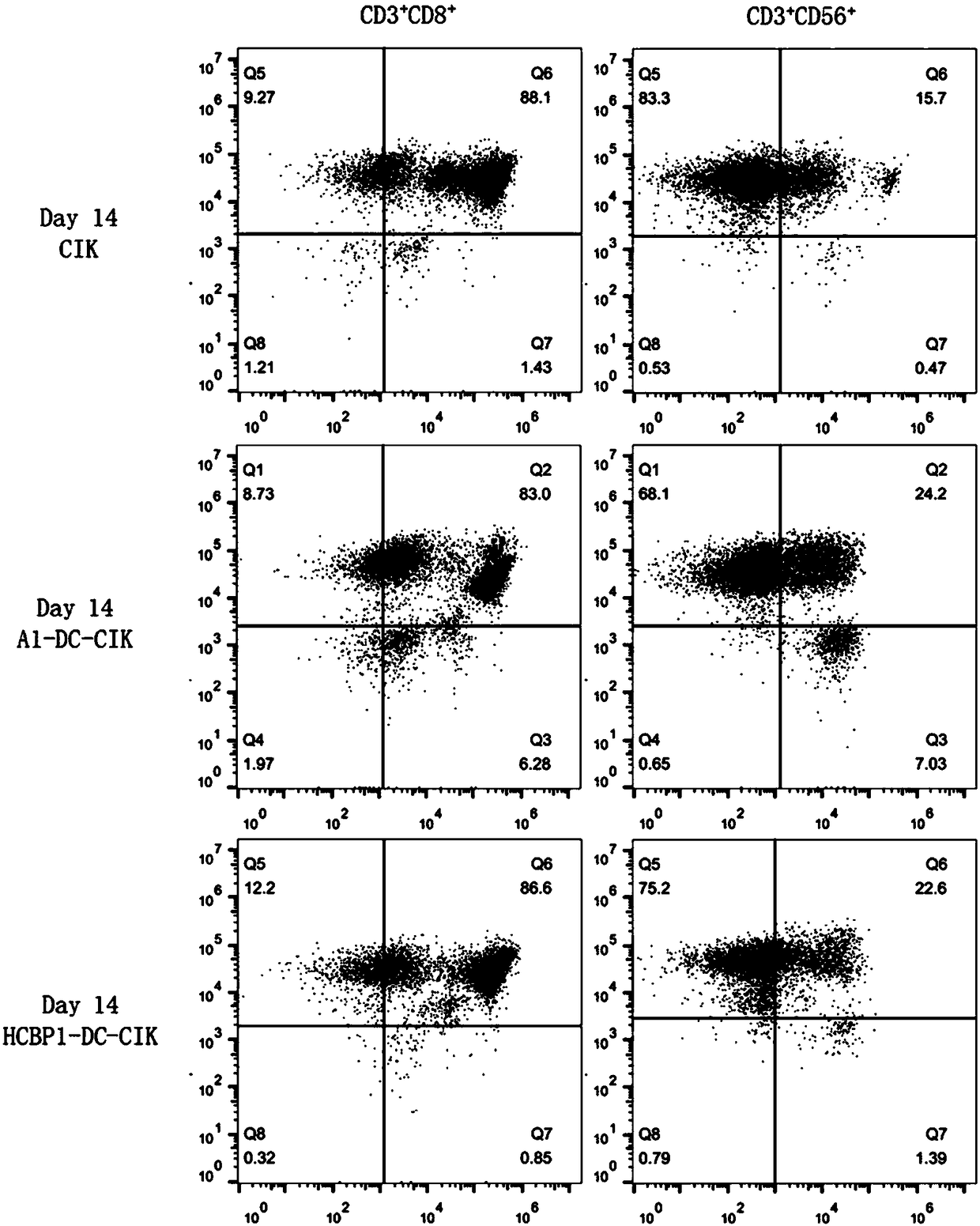
[0061]Embodiment 1: the culture of A1-DC-CIK cell (can refer to figure 1 shown)
[0062] 1. Isolation of mononuclear cells
[0063] 1) Take 20ml of peripheral blood from healthy volunteers, anticoagulate with heparin, and centrifuge at 400-600g for 20-40min at room temperature.
[0064] 2) After centrifugation, carefully draw the upper layer liquid with a pipette gun, stop when it is about 0.5 cm away from the buffy coat layer containing mononuclear cells, and discard the upper layer liquid.
[0065] 3) Carefully transfer the buffy coat to a new centrifuge tube with a pipette gun, add an appropriate amount of PBS to the centrifuge tube, and add more liquid than the buffy coat. Centrifuge at 200-300g for 5-10 minutes.
[0066] 4) Discard the supernatant, resuspend the cells with 5-10ml PBS, blow and wash gently with a pipette gun, and centrifuge at 200-300g for 5-10min.
[0067] 5) Repeat 4), discard the supernatant, resuspend the cells with RPMI-1640 complete medium contai...
Embodiment 2
[0092] Embodiment 2: the culture of HCBP1-DC-CIK cell (see figure 1 shown)
[0093] 1. Isolation of mononuclear cells
[0094] 1) Take 20ml of peripheral blood from healthy volunteers, anticoagulate with heparin, and centrifuge at 400-600g for 20-40min at room temperature.
[0095] 2) After centrifugation, carefully draw the upper layer liquid with a pipette gun, stop when it is about 0.5 cm away from the buffy coat layer containing mononuclear cells, and discard the upper layer liquid.
[0096] 3) Carefully transfer the buffy coat to a new centrifuge tube with a pipette gun, add an appropriate amount of PBS to the centrifuge tube, and add more liquid than the buffy coat. Centrifuge at 200-300g for 5-10 minutes.
[0097] 4) Discard the supernatant, resuspend the cells with 5-10ml PBS, blow and wash gently with a pipette gun, and centrifuge at 200-300g for 5-10min.
[0098] 5) Repeat 4), discard the supernatant, resuspend the cells with RPMI-1640 complete medium containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com