Bifunctional molecule combining CD3 and T-cell positive costimulatory molecules and application of bifunctional molecule
A bifunctional molecule and co-stimulatory molecule technology, applied in the field of biomedicine, can solve the problems of T cell incapacity, T cell death, and inability to effectively activate T cells, and achieve less protein consumption, simple purification process, in vitro activation and Amplify the effect of better effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0138] The method for preparing the aforementioned bifunctional molecule of the present invention comprises: constructing an expression vector containing the gene sequence of the bifunctional molecule, then transforming the expression vector containing the gene sequence of the bifunctional molecule into a host cell to induce expression, and isolating the expression product to obtain the Bifunctional molecules described above. In a preferred case of the present invention, the expression vector uses pcDNA3.1. The host cell is Chinese hamster ovary cell (CHO).
[0139] 6. Uses of Bifunctional Molecules
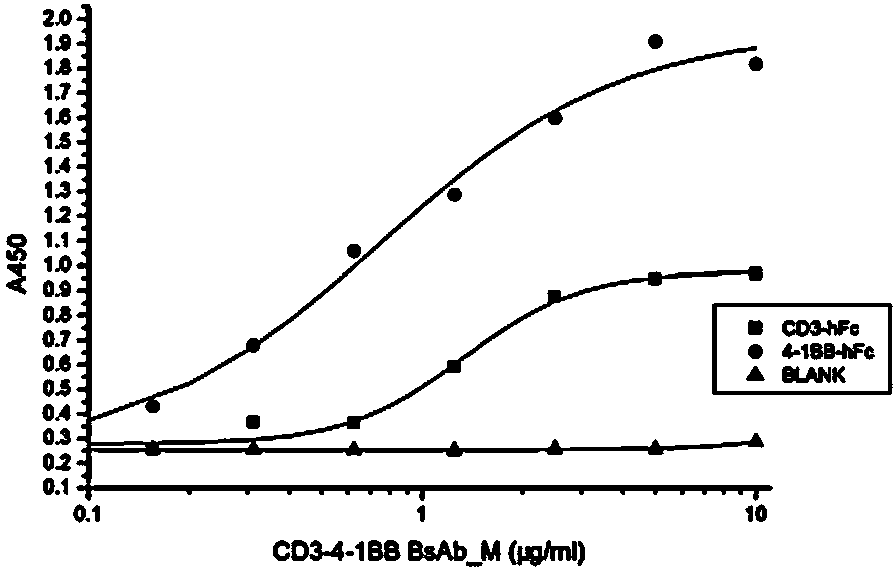
[0140] The bifunctional molecule of the invention can be used to prepare T cell expansion agent in vitro. In a preferred embodiment of the present invention, it is proved by experiments that the bifunctional molecules in the form of monomers and dimers have in vitro binding activity to CD3 and positive co-stimulatory molecule recombinant antigens, and can be applied to T cell a...
Embodiment 1
[0147] Example 1 Construction of CD3-4-1BB BsAb_M and CD3-4-1BB BsAb_D eukaryotic expression vectors
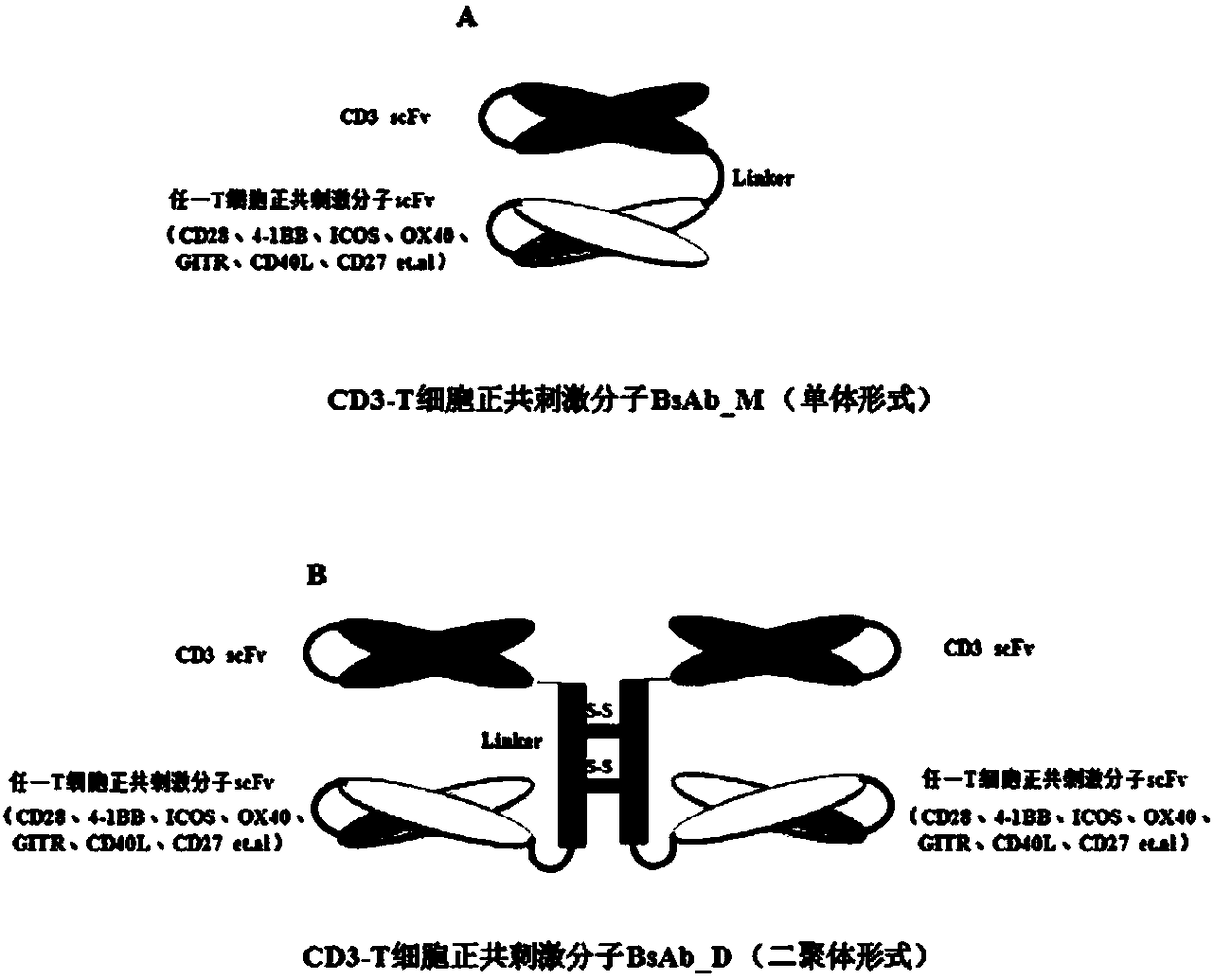
[0148] In the present invention, the bispecific antibody targeting the human CD3 protein on the surface of T cells and the positive co-stimulatory molecule 4-1BB protein on T cells is named CD3-4-1BB BsAb.
[0149] 1. CD3-4-1BB BsAb_M and CD3-4-1BB BsAb_D construction scheme design
[0150] The specific construction scheme of CD3-4-1BB BsAb_M in monomeric form is as follows: pass (GGGGS) between anti-CD3 scFv and anti-4-1BB scFv sequences 3 Linker is connected.
[0151] The specific construction scheme of the CD3-4-1BB BsAb_D in dimer form is as follows: the anti-CD3 scFv and anti-4-1BB scFv sequences are connected through the IgD hinge region as a Linker.
[0152] In order to express the bispecific antibody in mammalian cells, the sequences of anti-CD3 scFv, anti-4-1BB scFv and linker (Linker) were codon optimized for expression in mammalian system.
[0153] Specifically...
Embodiment 2
[0183] Example 2: Expression and purification of CD3-4-1BB BsAb_M and CD3-4-1BB BsAb_D
[0184] 1. Expression of CD3-4-1BB BsAb_M and CD3-4-1BB BsAb_D
[0185] 1.1. The passage density of CHO-S cells (purchased from Thermo Fisher Scientific) 1 day before transfection was 0.5-0.6×10 6 / ml;
[0186] 1.2. Count the cell density on the day of transfection, when the density is 1~1.4×10 6 / ml, when the activity is >90%, it can be used for plasmid transfection;
[0187] 1.3. Preparation of transfection complex: For each item (CD3-4-1BB BsAb_M and CD3-4-1BB BsAb_D), two centrifuge tubes / bottles need to be prepared, take 20ml as an example, place them separately, and take the samples in Example 1 Prepare recombinant plasmids:
[0188] Add 600μl PBS and 20μg recombinant plasmid to tube ①, mix well;
[0189] Add 600μl PBS, 20ul FreeStyle to tube ② TM MAX Transfection Reagent (purchased from Thermo Fisher Scientific company), mixing;
[0190] 1.4. Add the diluted transfection reag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Passage density | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
| Theoretical molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com