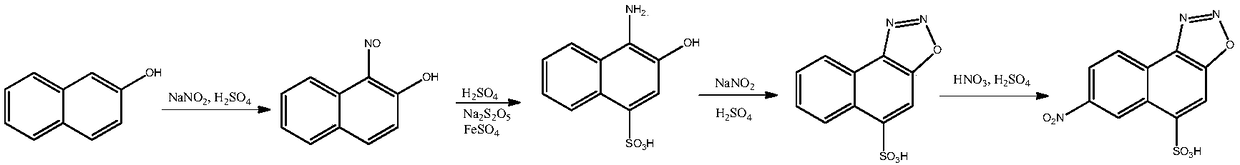
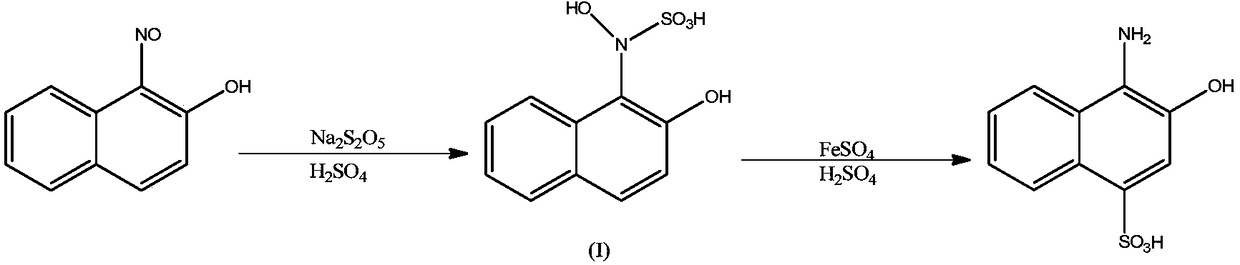
Naphthyl ammonium salt compound as well as preparation method and application thereof
A naphthylammonium salt and compound technology, which is applied in the field of purification of organic compounds, can solve the problem that the addition reaction product affects the quality of downstream products and the quality of 6-nitro-1,2-diazooxy-4-naphthalenesulfonic acid Can not meet the market demand and other problems, to achieve the effect of improving environmental compatibility, reducing process costs, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
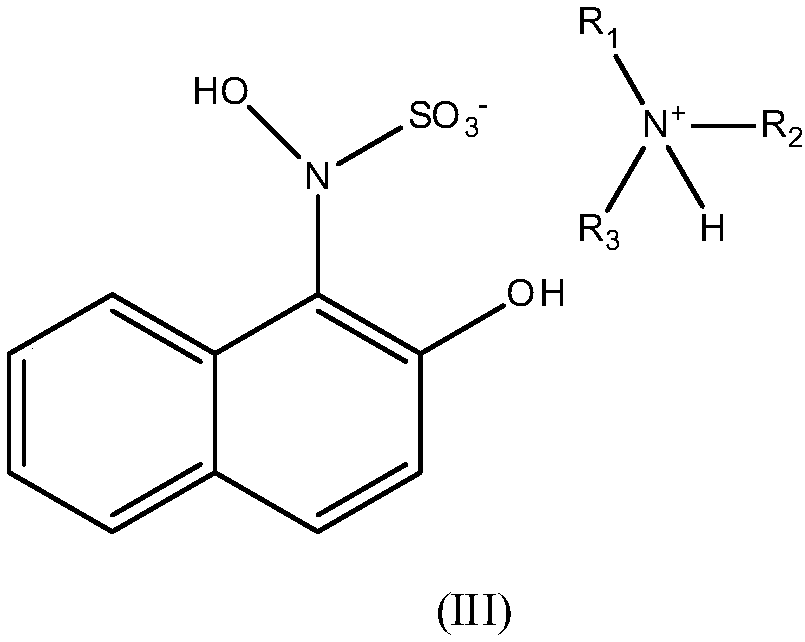
[0034] The naphthyl compound crude product shown in 2.55 kilograms of structural formula (I) is mixed with 12.75 kilograms of acetone, 1.9 kilograms of dicyclohexylamines, is stirred until reaction finishes, suction filtration, obtains 4.22 kilograms (yield 98.4%, HPLC purity 99.5%) structural formula The naphthyl ammonium salt compound shown in (III), the NMR data of the naphthyl ammonium salt compound shown in gained structural formula (III): 1 HNMR (500MHz, DMSO-d 6 ): δ8.08(d,1H), 8.01(d,1H), 7.77(t,1H), 7.56(t,1H), 7.43(d,1H), 7.05(d,1H), 6.89(br, 2H), 5.44 (br, 1H), 3.55 (m, 2H), 2.02-2.27 (m, 8H), 1.92 (br, 1H), 1.43-1.54 (m, 12H).
[0035] 4.22 kilograms (yield 98.4%, HPLC purity 99.5%) of naphthyl ammonium salt compound shown in structural formula (III) obtained through the above-mentioned process are mixed with 12.87 kilograms of water, add sodium hydroxide to adjust to system pH to 13, reclaim organic alkali to obtain an alkaline aqueous solution; the resulting al...
Embodiment 2
[0037] The naphthyl compound crude product shown in 2.55 kilograms of structural formula (I) is mixed with 12.75 kilograms of acetone, 1.01 kilograms of triethylamine, is stirred to the end of reaction, and suction filtration obtains 3.4 kilograms (yield 96.6%, HPLC purity 98.7%) structural formula ( III) shown in the naphthyl ammonium salt compound, the nuclear magnetic resonance spectrum data of the naphthyl ammonium salt compound shown in the gained structural formula (III): 1 HNMR (500MHz, DMSO-d 6 ): δ8.01~8.03(t,2H), 7.31~7.55(t,2H), 7.27(d,1H), 7.05(d,1H), 7.01(d,1H), 5.34(br,1H), 3.25(q,4H), 1.57(t,6H).
[0038] 3.4 kg (yield 96.6%, HPLC purity 98.7%) obtained through the above-mentioned process are mixed with 17 kg of water with the naphthyl ammonium salt compound shown in structural formula (III), and the system uses sulfuric acid to adjust the system pH=3 to obtain 2.43 kg ( Yield 94.2%, HPLC purity 99.1%) the naphthyl compound refined product shown in structural ...
Embodiment 3
[0040] The naphthyl compound crude product shown in 2.55 kilograms of structural formulas (I) is mixed with 7.65 kilograms of water, 1.01 kilograms of triethylamine, is stirred to the end of reaction, and suction filtration obtains 3.4 kilograms (yield 96.6%, HPLC purity 98.7%) structural formula ( III) shown in the naphthyl ammonium salt compound, the nuclear magnetic resonance spectrum data of the naphthyl ammonium salt compound shown in the gained structural formula (III): 1 HNMR (500MHz, DMSO-d 6 ): δ7.97~8.01(d,2H), 7.38~7.45(t,2H), 7.28(d,1H), 7.03(d,1H), 7.01(d,1H), 5.30(br,1H), 4.01(q,2H), 1.60(t,12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com