A kind of anti-activated blood coagulation factor V monoclonal antibody and its preparation method and application
A monoclonal antibody and coagulation factor technology, applied in the biological field, can solve the problems of no anti-activated FV monoclonal antibody preparation report, lack of quantitative detection of activated FV and other problems, and achieve the effect of high affinity, strong specificity and good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1 Preparation of monoclonal antibody against activated FV of the present invention
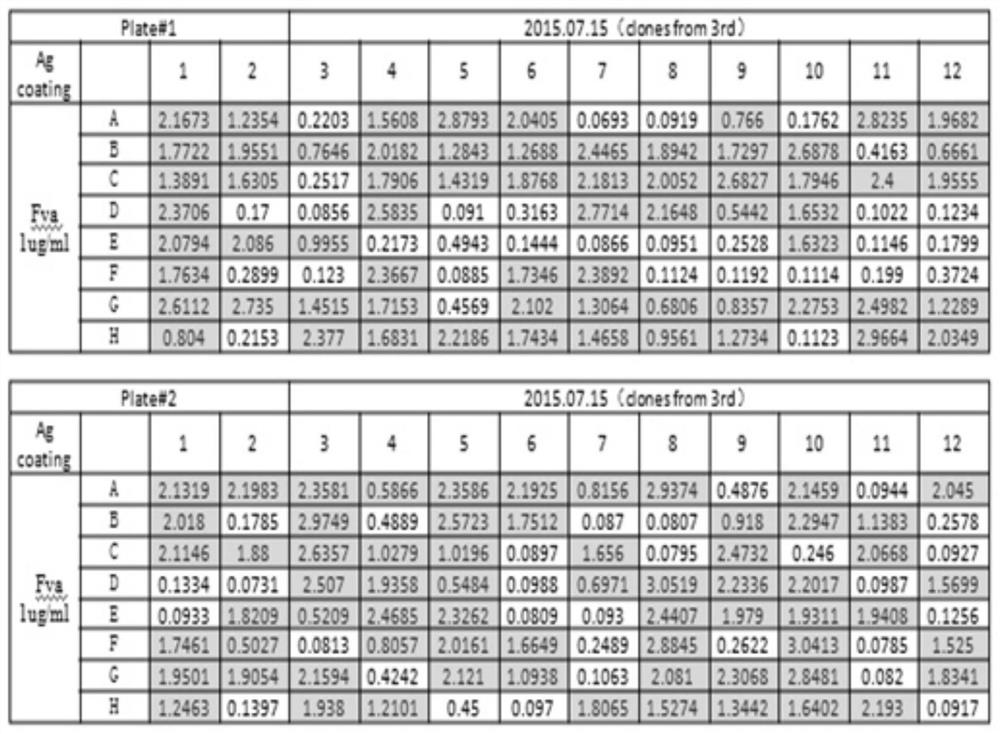
[0022] 1. Screening of fully human FVa single chain antibody in phage antibody library
[0023] The FVa recombinant protein (product of HTI Company) was used as an antigen to screen the fully human phage antibody library. Dilute the FVa antigen with coating diluent (0.05mol / L sodium carbonate-sodium bicarbonate, pH 9.6) to 10ug / mL, add 100ul to each well of the ELISA plate, and place in a 4°C refrigerator for overnight coating. The blocking solution was mixed with PBST (PBS, 0.05% Tween 20) and 1% BSA, and 200ul was added to each well, and blocked for 1 hour. Wash 5 times with PBST, 3 min each time, add fully human Fab-based phage display library (from Shanghai Boyu Biotechnology Company phage display library), and incubate overnight at room temperature. Discard the liquid in the immunoplate, wash with PBST 5 times, each time for 3 minutes, and the phage tightly bound to th...
experiment example 1
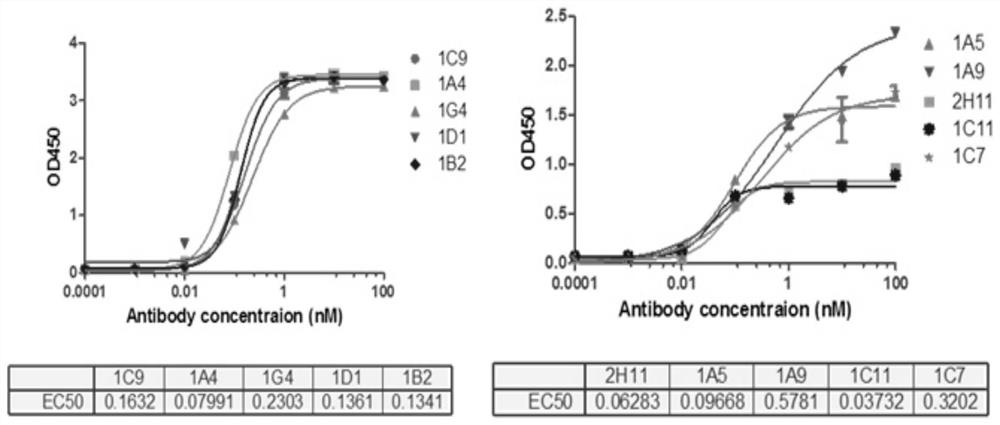
[0040] Experimental example 1 Antigen-binding activity assay of the monoclonal antibody of the present invention
[0041] 1. Experimental method
[0042] The purified monoclonal antibody 1C11 prepared in Example 1 was used for gradient concentration affinity detection by ELISA method.
[0043] ELISA method: Dilute the FVa antigen with coating diluent to 1ug / mL, add 100ul of the diluted antigen to each well of the ELISA plate, and place it in a 4°C refrigerator overnight. Add 150ul of blocking solution to each well, and block at 37°C for 40min. Add 200ul of washing solution to each well and wash 3 times, each time 3min. Add the monoclonal recombinant phage to be tested and incubate at 37°C for 2h. Wash 3 times with washing solution, 3 min each time. Add goat anti-human-HRP (dilution ratio 1:2000) working solution, incubate at 37°C for 1 hour, add 200ul washing solution to each well and wash 3 times, 3 minutes each time. Add 100ul of temporarily prepared TMB substrate solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com