Pd-1 antibodies and uses thereof
A technology of PD-1 and antibody, which is applied in the field of immunotherapy for the treatment of human diseases, and can solve problems such as the lack of MYPPPY motifs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0142] Example 1. Construction and expression of PD-1-Fc and PD-L1-Fc
[0143]A DNA (SEQ ID NO.11) containing cDNA encoding the extracellular region of human PD-1 fused to human Fc with a Hind III site at its 5' end and an EcoRI site at its 3' end was synthesized. This DNA was digested with Hind III and EcoRI and then cloned into the pcDNA3.1(+) vector (Invitrogen) which had been digested with the same restriction enzymes, resulting in the expression vector pcDNA3.1(+)-PD-1-Fc. The appropriate pcDNA3.1(+)-PD-1-Fc expression vector was transfected into CHO-K1 cells (ATCC) using Lipofectamine 2000 reagent (Invitrogen). Stable transfectants were isolated by limiting dilution in the presence of 500 μg / ml G418. The clone producing the highest amount of PD-1-Fc protein was selected and grown in serum-free medium. Finally, PD-1-Fc protein was purified from serum-free culture supernatant by protein A affinity chromatography. Protein concentration was determined by absorbance at 280...
Embodiment 2
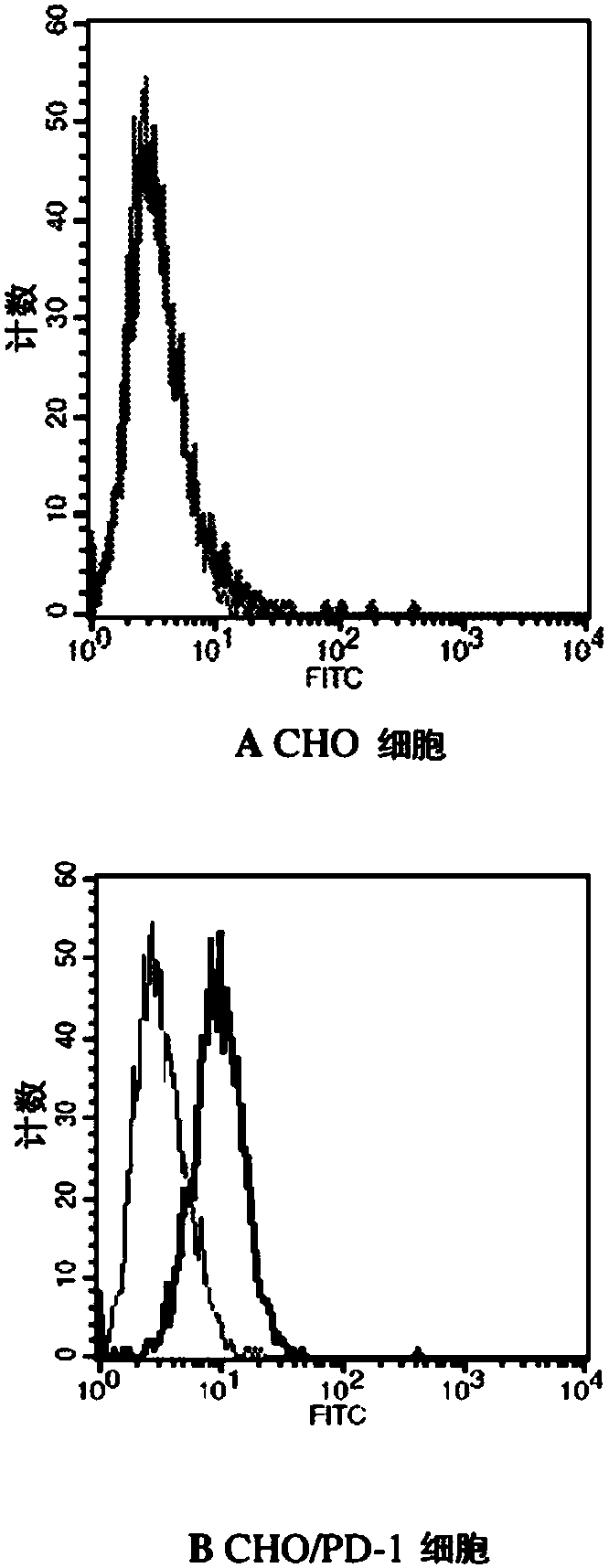
[0145] Example 2. Characterization of PD-L1-Fc binding to PD-1 expressed on CHO cells
[0146] A Chinese hamster ovary (CHO) cell line expressing recombinant human PD-1 on the cell surface was developed and used to determine the binding of PD-L1 to PD-1 by flow cytometry. A DNA (SEQ ID NO. 13) containing a cDNA encoding full-length human PD-1 with a Hind III site at its 5' end and an EcoRI site at its 3' end was synthesized. This DNA was digested with Hind III and EcoRI and then cloned into the pcDNA3.1(+) vector (Invitrogen) which had been digested with the same restriction enzymes, resulting in the expression vector pcDNA3.1(+)-PD-1. The appropriate pcDNA3.1(+)-PD-1 expression vector was transfected into CHO-K1 cells (ATCC) using Lipofectamine 2000 reagent (Invitrogen). Stable transfectants were isolated by limiting dilution in the presence of 500 μg / ml G418. The amino acid sequence of full length human PD-1 is shown in SEQ ID NO:14. The PD-L1-Fc recombinant protein was l...
Embodiment 3
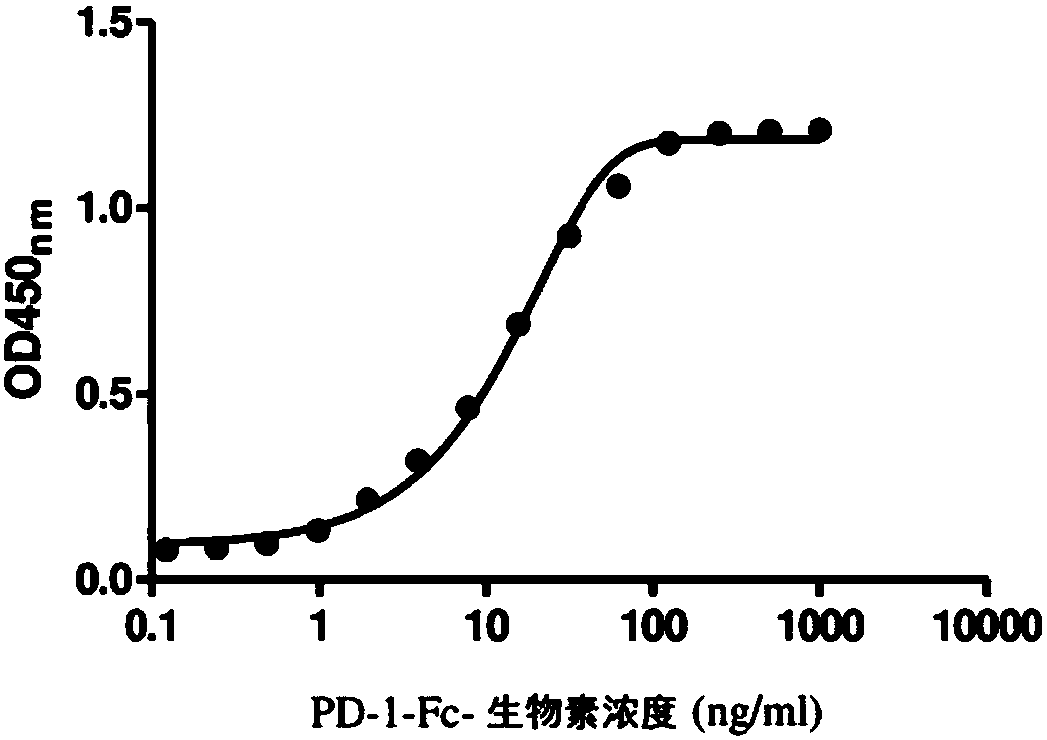
[0147] Example 3. Characterization of PD-1-Fc binding to PD-L1-Fc
[0148] The PD-1-Fc recombinant protein was labeled with biotin using the Biotin protein labeling kit (Roche). Different concentrations of biotin-labeled PD-1-Fc (PD-1-Fc-biotin) were added to the 96-well plate coated with PD-L1-Fc, followed by incubation at room temperature for 1 h. After washing, avidin-HRP was added and the plates were further incubated for 1 h. Finally, 3,3',5,5'-tetramethylbenzidine (TMB) was added as a substrate for horseradish peroxidase (HRP), and the absorbance was measured at 450 nm. figure 2 The results shown in indicate that biotin-PD-1-Fc can effectively bind PD-L1-Fc.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com