Chlormadinone acetate self-microemulsion composition and preparing method and application thereof
A technology of chlormadinone and self-microemulsion, which is applied in the direction of drug combination, pharmaceutical formula, emulsion delivery, etc., can solve the problem of low curative effect, reduce the dosage, improve the compliance of use, and improve the oral bioavailability degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
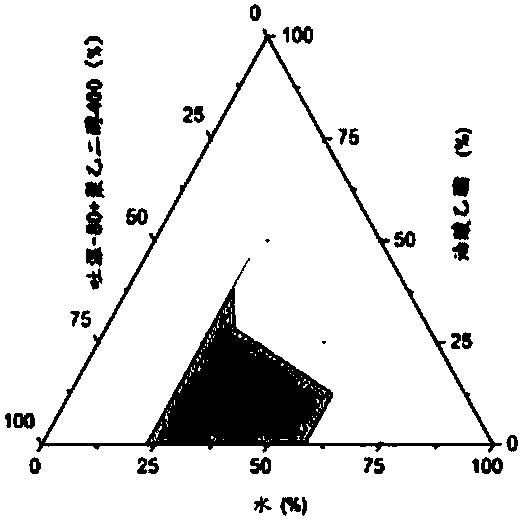
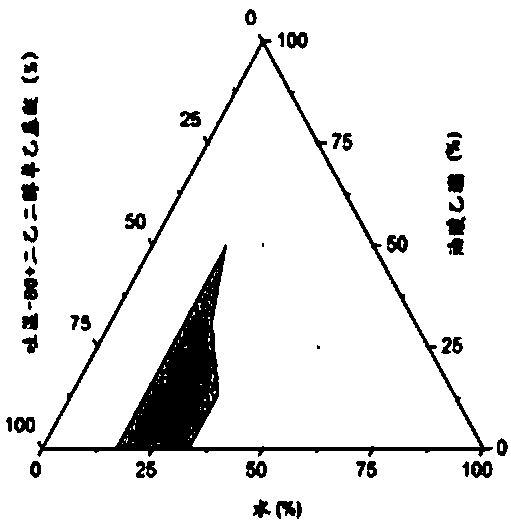
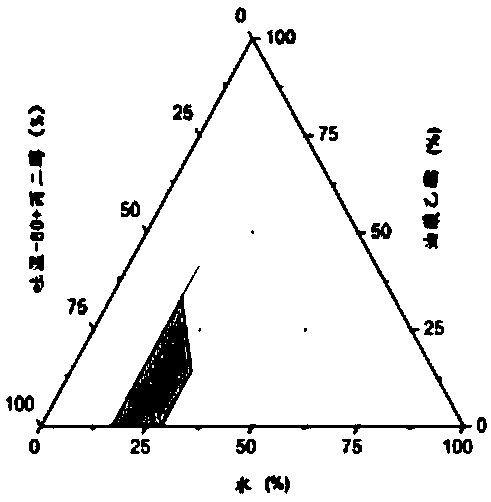
Image
Examples
Embodiment 1
[0043] The pre-prescription study of embodiment 1 chlormadinone self-microemulsion composition
[0044] 1. Determination of equilibrium solubility of chlormadinone in each auxiliary material
[0045] Take 2ml each of the oil phase, surfactant and co-surfactant into a 10ml stoppered test tube, add excess chlormadinone raw material (about 50mg) respectively, vortex and mix well, and shake in a constant temperature water bath shaker at 37°C 72h (110rpm) to reach the dissolution equilibrium, take 1ml to 1.5ml EP tube, centrifuge (15000rpm, 10min), the supernatant is diluted with isopropanol, HPLC sample analysis, determination of chlormadinone in each oil phase, surface The equilibrium solubility in active agent and cosurfactant, measurement result is shown in Table 1. The results showed that different excipients had different dissolving abilities to chlormadinone, and the dissolution order of each oil relative to chlormadinone was as follows: Labrafac Lipophile WL 1349>oleic aci...
Embodiment 2
[0073] Embodiment 2 The preparation of self-microemulsion composition
Embodiment 21
[0075] Accurately weigh 100 mg of chlormadinone raw material, add 6 g of diethylene glycol monoethyl ether to dissolve the drug, and then add 8 g of polyethylene glycol 400, 20 g of Tween-80 and 16 g of ethyl oleate in sequence under magnetic stirring , after forming a transparent and uniform self-microemulsion composition, divide it into 50 parts on average, each part is equivalent to containing 2 mg of the main drug chlormadinone, which is equivalent to a single dose of the original drug The amount of chlormadinone contained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com