Full-length infectious DNA (deoxyribonucleic acid) clone of porcine parvoviruses, method for constructing full-length infectious DNA and application thereof
A parvovirus and infectious technology, applied in the field of the full-length infectious clone of porcine parvovirus and its construction, the virus infectious clone and its construction, and the establishment of the reverse genetics operating system of porcine parvovirus, which can solve the difficult problem. Duplication, no virus rescue pathogenic mechanism, influence on the establishment of reverse genetics operation platform, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Construction of mutant porcine parvovirus vector
[0039] (1) Transformation vector pBluscript II SK(+) (purchased from Bao Biological Engineering Co., Ltd.):
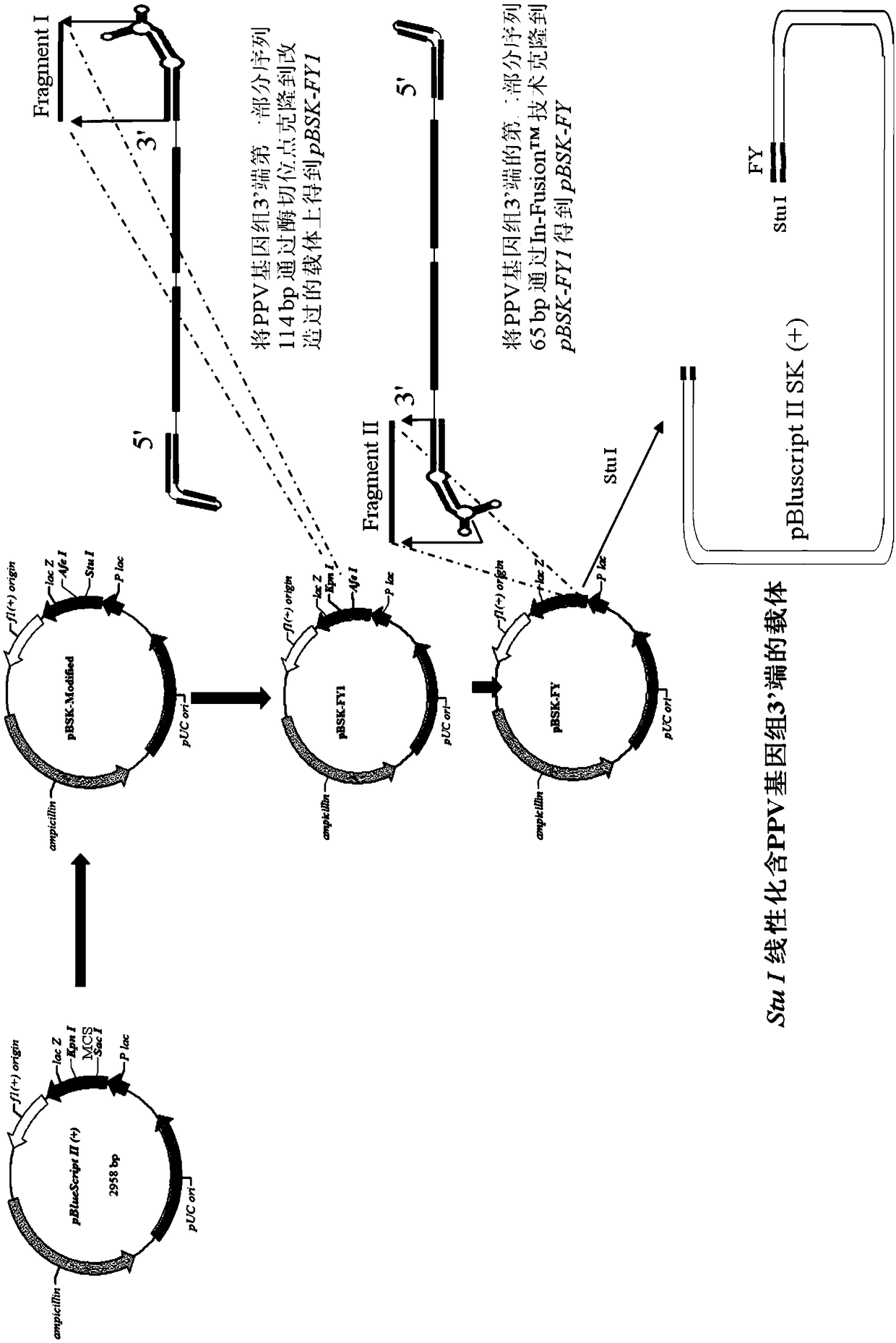
[0040] Add two restriction sites, namely Afe I and Stu I, between the Kpn I and Sac I restriction sites in the vector pBluscript II SK(+), to obtain a new multi-cloning restriction site Kpn I-Afe I -Stu I-Sac I, the modified vector is named pBSK-Modified;
[0041] (2) Synthesis of full-length infectious DNA of porcine parvovirus
[0042] Synthesize a 114bp fragment (fragment I) at the 3' end of the PPV genome, and clone it into the transformed pBSK-Modified vector through the Kpn I / Afe I restriction site to obtain pBSK-FY1; synthesize PPV genome 3 linked to fragment I The fragment (fragment II) with a length of 65 bp at the 'end was ligated with fragment I by Infusion cloning technology to obtain pBSK-FY; the vector containing the 3' end fragment of the PPV genome was linearized with Stu I; it was de...
Embodiment 2
[0045] The preparation of embodiment 2 rescue virus
[0046] PK-15 cells were inoculated and placed in a six-well plate, and transfected when the cell density was 80%-90%. 5ug of plasmid pPPV and 10ul of Lipofectamine 2000 (purchased from Invitrogen) transfection reagent were added to each well, shaken and mixed in 500ul of Opti-MEM, and transfected according to the instructions of the transfection reagent. And observe the cytopathic changes day by day. Cytopathic changes (CPE) appeared on the third day. On the fifth day, the cell transfection was collected and frozen and thawed three times and blindly passed. The virus cell culture of the seventh generation was harvested and frozen and thawed repeatedly three times to obtain the rescued virus pPPV, which was stored at -70°C for future use.
Embodiment 3
[0047] The identification of embodiment 3 rescue virus
[0048] The rescue virus prepared in Example 2 was tested for titer with the parental virus, and the results showed that there was no significant difference between the parental virus PPV and the rescued virus pPPV TCID50 and hemagglutination titer, and the multi-step growth curve was measured at the same time, and the results showed that 8h after infection After the start of replication, the replication peak was reached at 60h, and the difference between the wild virus and the rescued virus was not significant ( image 3 ).
[0049] The results show that the present invention uses the reverse genetic operating system to obtain a full-length infectious clone with similar growth characteristics to the wild virus, and at the same time shows that the introduction of biogenetic markers into the virus genome does not affect the growth of the entire virus. The genetic marker EcoRv introduced into the full-length infectious clo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com