Iron-catalyzed cyanide alkylcyanoalkyl indoline and preparation method thereof
A cyanoalkyl indoline and cyclization technology, applied in the field of chemical materials, can solve problems such as large steric hindrance and no reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] In order to make the object, technical solution and advantages of the present invention more clear, the present invention will be further described in detail below in conjunction with the examples. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
[0032] The application principle of the present invention will be further described below in conjunction with the accompanying drawings.
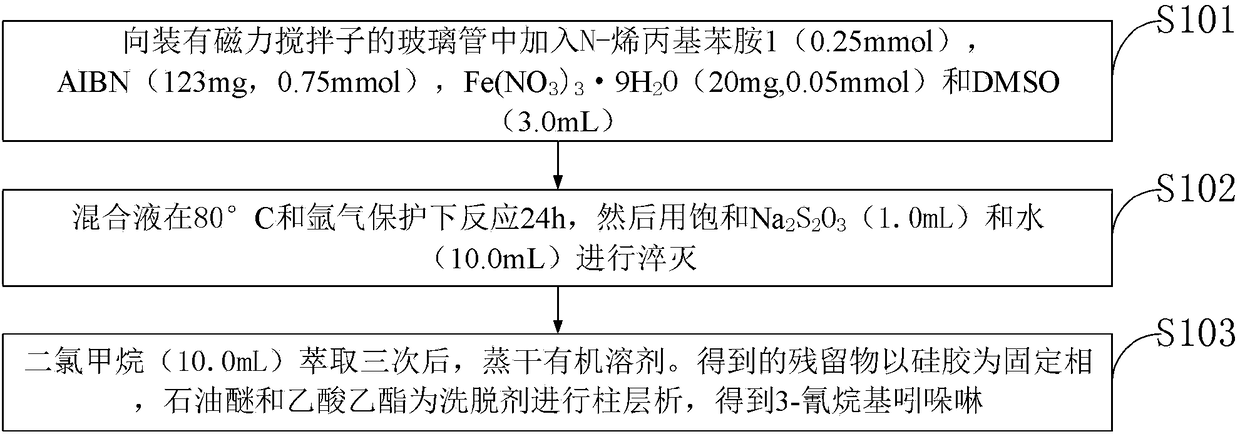
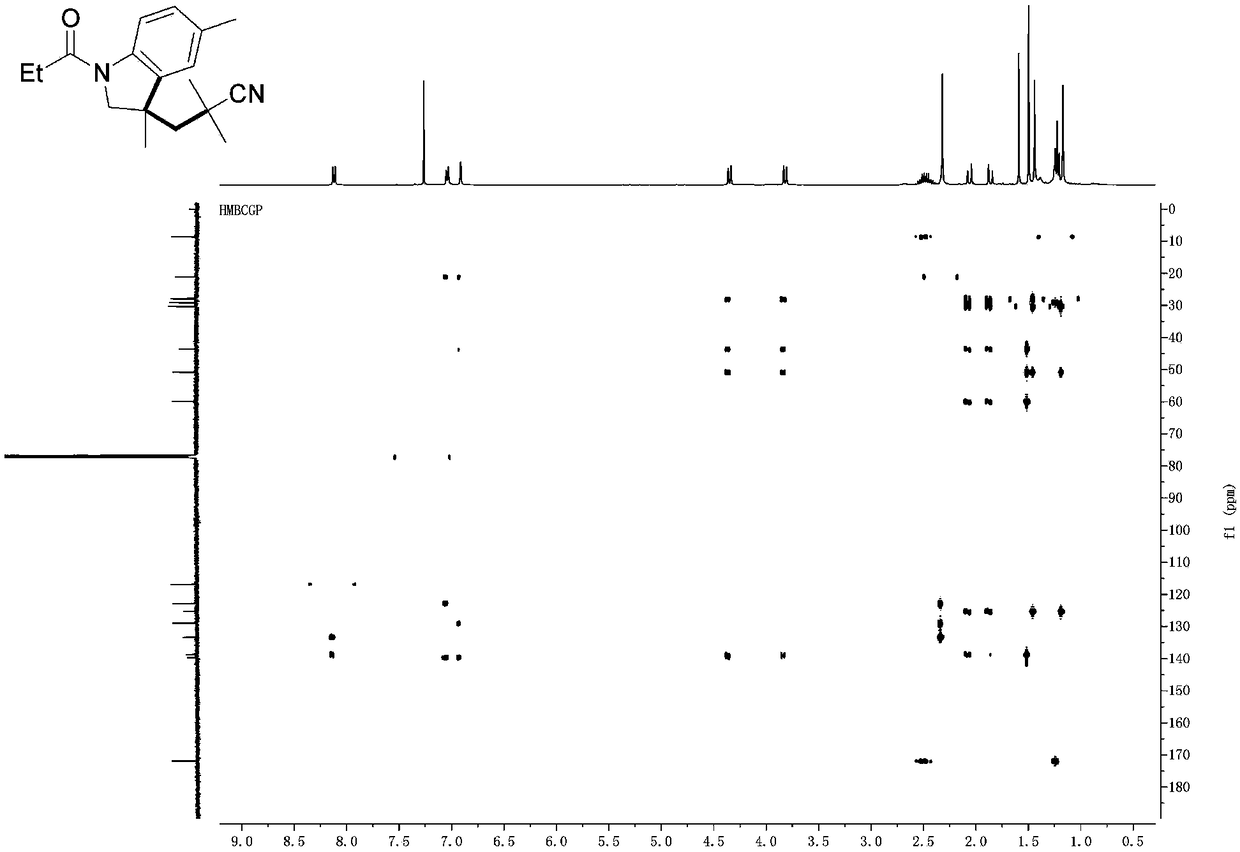
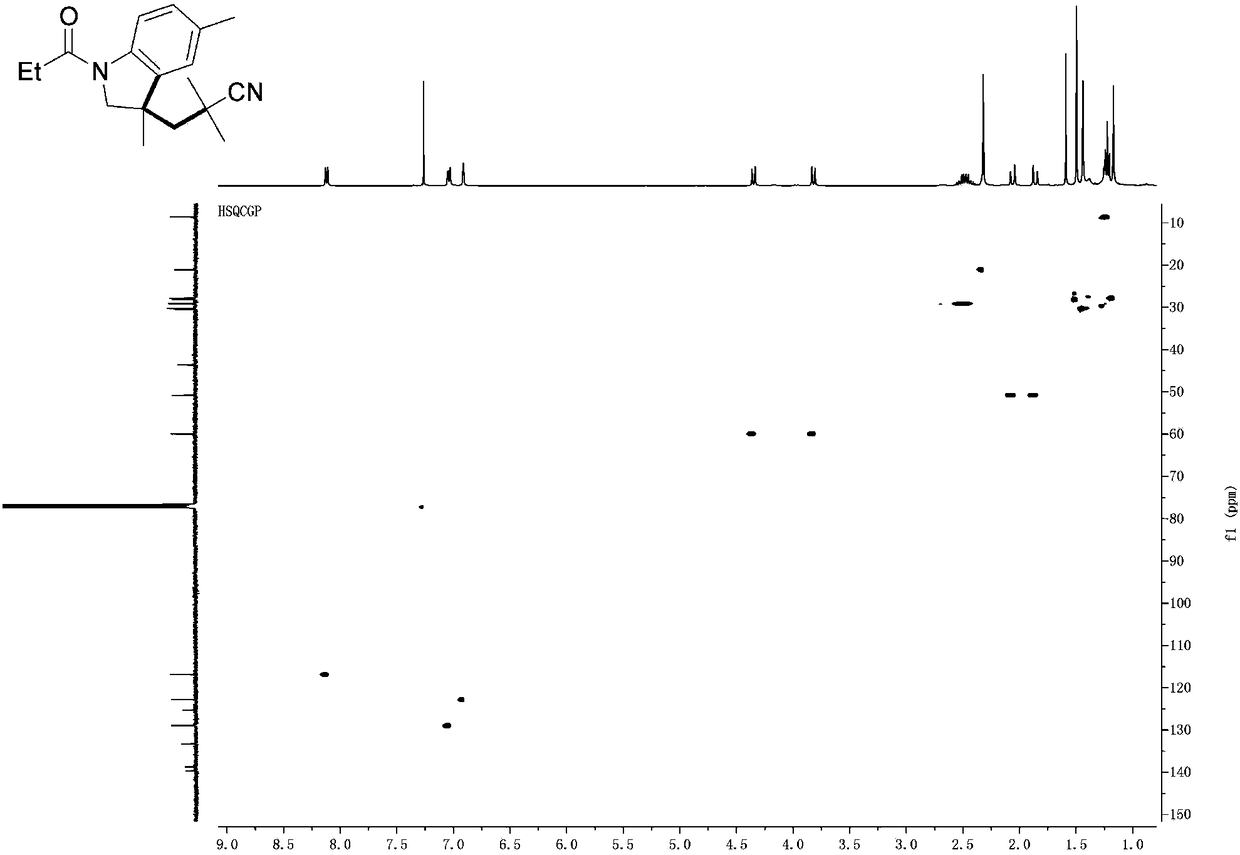
[0033] Such as figure 1 As shown, the iron-catalyzed cyanoalkyl indoline and preparation method thereof provided by the embodiments of the present invention comprise the following steps:
[0034] S101, add N-allylaniline 1 (0.25mmol), AIBN (123mg, 0.75mmol), Fe(NO 3 ) 3 9H 2 O (20 mg, 0.05 mmol) and DMSO (3.0 mL);
[0035] S102, the mixed solution was reacted at 80°C under the protection of argon for 24h, and then saturated Na 2 S 2 o 3 (1.0mL) and water (10.0mL) were quenched; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com