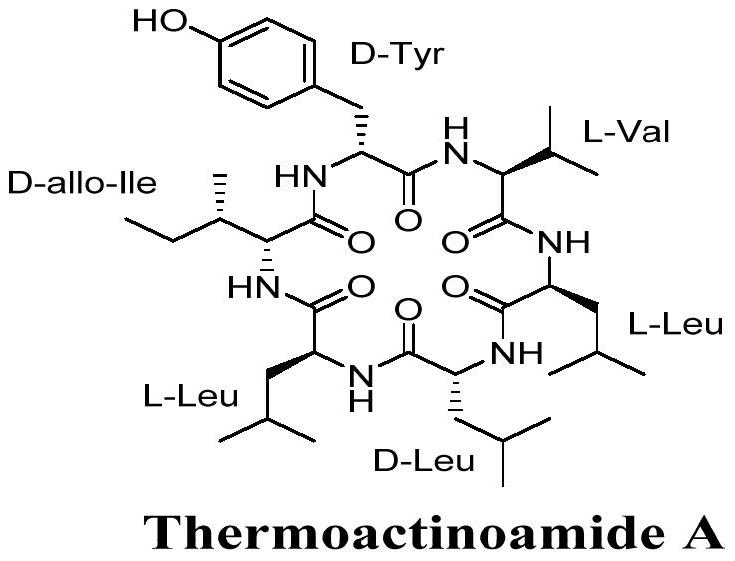
A kind of synthetic method of antibacterial activity cyclic hexapeptide thermoactinoamide A
A synthesis method and technology of antibacterial activity, applied in the field of synthesis of antibacterial active cyclohexapeptide Thermoactinoamide A, can solve the problem that the output cannot meet the practical application and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
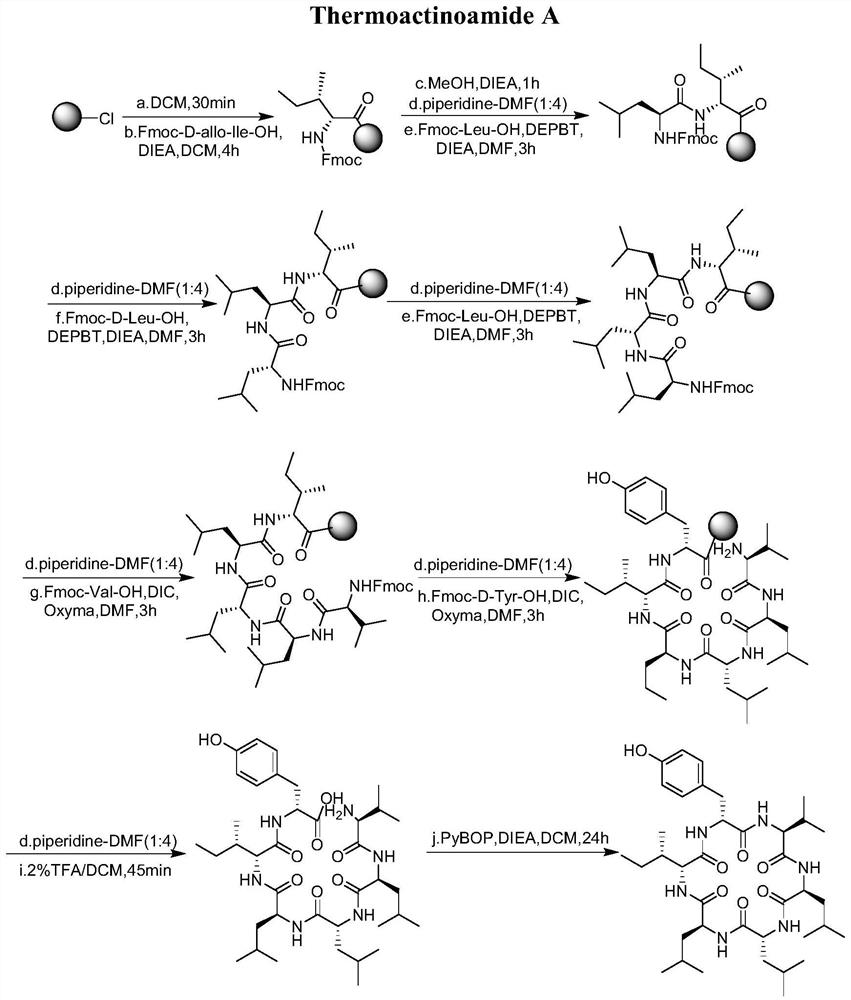
[0048] Embodiment 1: Resin complex of fluorenylmethoxycarbonyl isoleucine (Fmoc-D-allo-Ile-resin)
[0049] Take 1.1 g of 2-chlorotriphenylmethyl chloride resin (loading capacity: 0.94 mmol / g) in a 100 ml peptide synthesis tube, add 20 ml of dichloromethane (DCM) to swell for 30 minutes. The filtrate was drained, and fluorenylmethoxycarbonyl D-isoleucine (Fmoc-D-allo-Ile-OH, 0.5g, 1.4mmol) and diisopropylethylamine (DIEA , 463uL, 2.8mmol), stirred or shaken for 4 hours, the filtrate was drained, washed with dichloromethane (DCM, 10ml) and dimethylformamide (DMF, 10ml) alternately for 3 times each, and the filtrate was drained to obtain Fmoc-D-allo-Ile-resin.
Embodiment 2
[0050] Example 2: Residual site blocking on resin
[0051] A methanol (MeOH, 18ml) solution with a volume fraction of 10% diisopropylethylamine (DIEA, 2ml) was added to the product of Example 1, stirred or shaken for 30min, the filtrate was drained, and 10ml of DCM was used to , DMF alternately washed 3 times, and the filtrate was drained.
Embodiment 3
[0052] Example 3: Protected linear dipeptide-resin complex (Fmoc-Leu-D-allo-Ile-resin)
[0053] (1) Add the DMF solution of the 20% piperidine of 10ml in the product of embodiment 2, react for 10 minutes, wash 3 times alternately with DCM, DMF respectively of 10ml, drain the filtrate, for more thorough removal of fluorenylmethoxy For carbonyl (Fmoc) protection, the steps before step (1) can be repeated again, that is, add 10 ml of 20% piperidine in DMF solution to the filter residue after the filtrate is drained, react for 10 minutes, and use 10 ml of DCM and DMF respectively Rinse 3 times alternately, drain the filtrate, and complete the deprotection;
[0054] (2) Weigh Fmoc-Leu-OH (Fmoc-Leu-OH, 0.99g, 2.8mmol) and 3-(diethoxy-o-acyloxy)-1,2,3-benzotriazine -4-ketone (DEPBT, 0.84g, 2.8mmol) in a 50ml conical flask, after adding 10ml of DMF to dissolve, then add diisopropylethylamine (DIEA, 0.46ml, 2.8mmol), add step ( 1) The product after draining the filtrate in the medium...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com