Quinophthalone compound, preparation method thereof and application of quinophthalone compound as light absorption material
A technology of light-absorbing materials and compounds, applied in organic chemistry and other fields, can solve the problems of loss of visible light, low transmittance of visible light, color distortion, etc., and achieve the effects of protecting eyesight, good absorption effect, and reducing impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
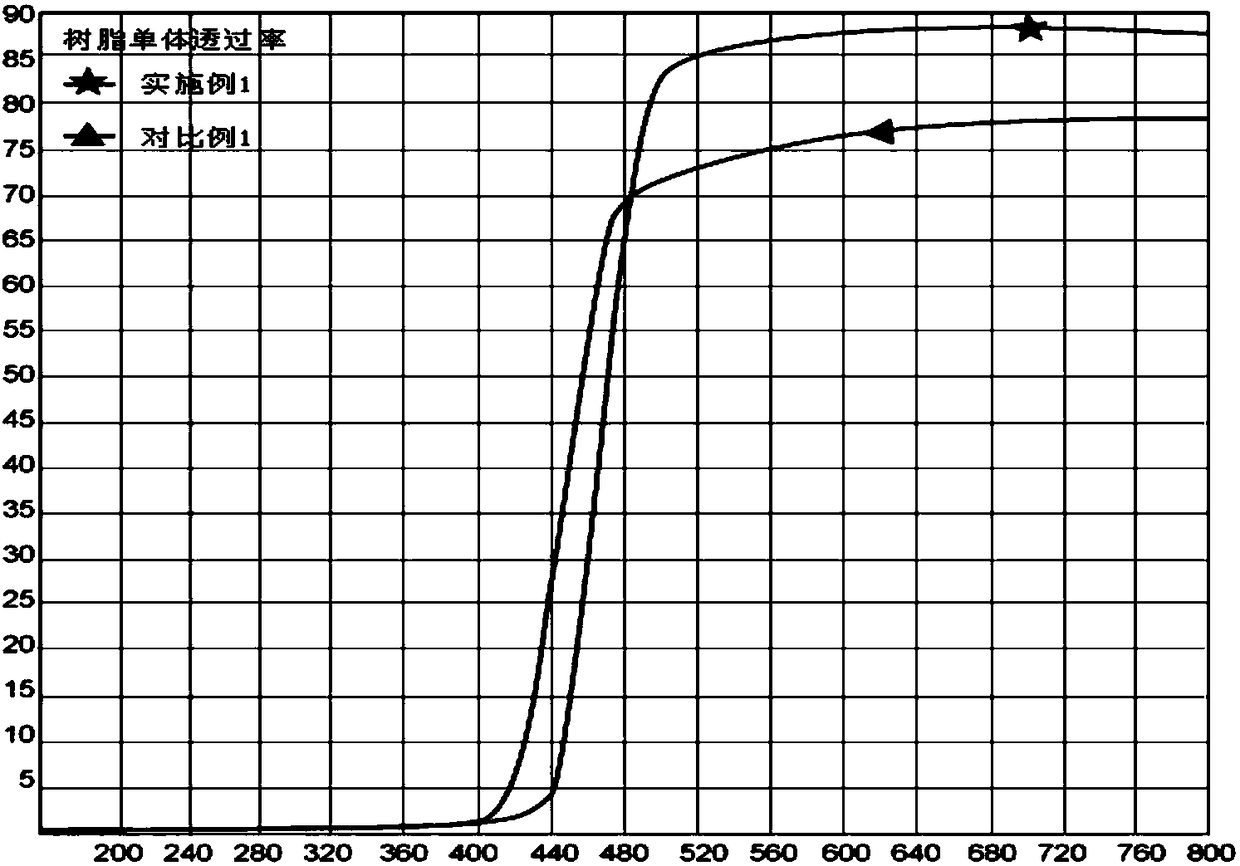
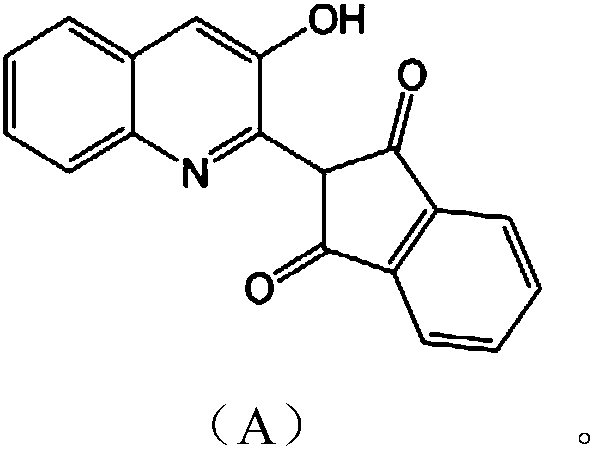
[0025] Embodiment 1: Preparation of quinophthalone compound (formula A), anti-blue light resin monomer and anti-blue light lens
[0026] (1) prepare quinophthalone compound (formula A), the chemical reaction equation is as follows:
[0027]
[0028] Add 100g of phthalic anhydride and 380g of 2-methyl-3-hydroxyquinoline into the reaction vessel, then add 380g of solvent 1,3,5-trichlorobenzene, slowly raise the temperature to 180°C, and at this temperature Carry out condensation reaction, keep it for 2 hours, lower the temperature to room temperature, filter the mixed product to obtain separate filtrate and filter cake, wash the filter cake with ethanol and dry it in a vacuum oven at 120°C for 1.5 hours to obtain a light yellow solid, which is ground After that, the quinophthalone compound was obtained, MS: 289.
[0029] (2) Preparation of anti-blue light resin monomer: After fully mixing and dissolving 1.0g quinophthalone compound (Formula A) and 12g dichloromethane, add it...
Embodiment 2
[0031] Embodiment 2: Preparation of quinophthalone compound (formula A), anti-blue light resin monomer and anti-blue light lens
[0032] (1) Preparation of quinophthalone compound (formula A): 100g phthalic anhydride, 300g 2-methyl-3-hydroxyquinoline are added to the reaction vessel, and then 260g solvent 1,3,5-trichloro Benzene, slowly raise the temperature to 180°C, and carry out the condensation reaction at this temperature, keep it for 2.5 hours, then lower the temperature to room temperature, filter the mixed product, obtain the separated filtrate and filter cake, wash the filter cake with ethanol and vacuum at 140°C After drying in a drying oven for 1 hour, a light yellow solid was obtained. After grinding, the quinophthalone compound (Formula A) was obtained, MS: 289.
[0033] (2) Preparation of anti-blue light resin monomer: After fully mixing and dissolving 1.0g quinophthalone compound (formula A) and 15g dichloromethane, add it into a reaction vessel containing 1400g...
Embodiment 3
[0035] Embodiment 3: Preparation of quinophthalone compound (formula A), anti-blue light resin monomer and anti-blue light lens
[0036] (1) Preparation of quinophthalone compound (formula A): 100g phthalic anhydride, 500g 2-methyl-3-hydroxyquinoline are added to the reaction vessel, and then 600g solvent 1,3,5-trichloro Benzene, slowly raise the temperature to 200°C, and carry out the condensation reaction at this temperature, keep it for 3 hours, then lower the temperature to room temperature, filter the mixed product, obtain the separated filtrate and filter cake, wash the filter cake with ethanol and vacuum at 100°C After drying in a drying oven for 2 hours, a light yellow solid was obtained. After grinding, the quinophthalone compound (Formula A) was obtained, MS: 289.
[0037] (2) Preparation of anti-blue light resin monomer: After fully mixing and dissolving 1.5g quinophthalone compound (Formula A) and 10g dichloromethane, add it to the reaction containing 800g allyl digl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thermal resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com