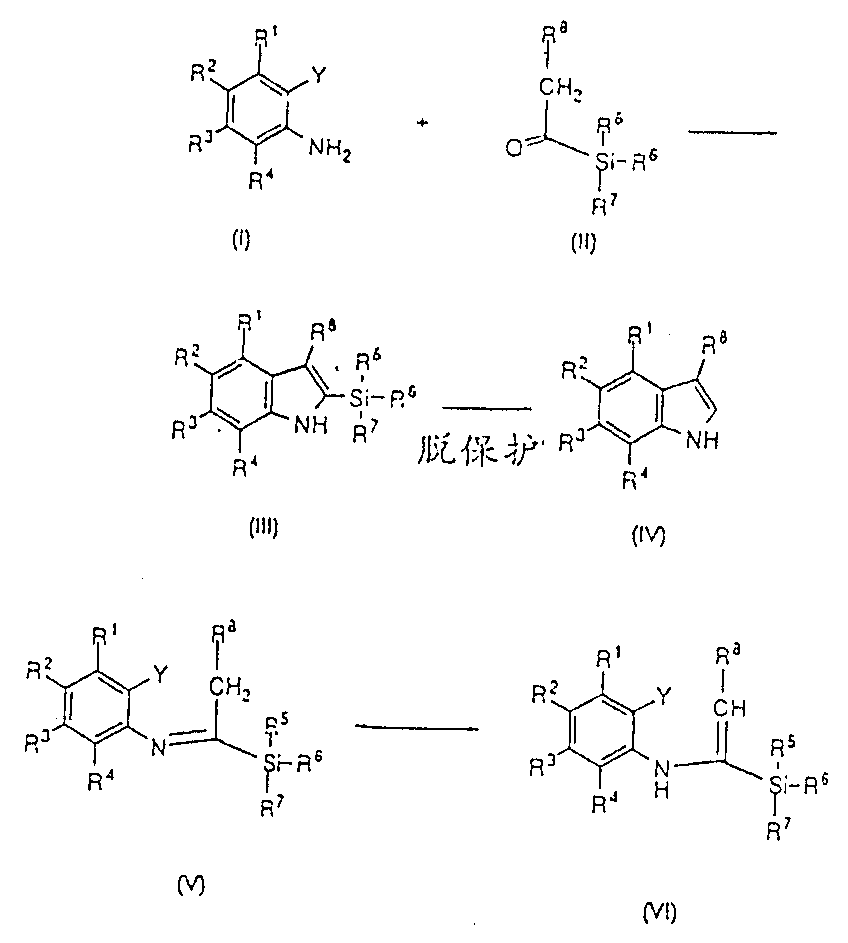
Palladium catalyzed indolization
A palladium catalyst and reaction technology, applied in organic chemistry, silicon organic compounds, bulk chemical production, etc., can solve expensive problems and achieve the effect of eliminating triazole-based polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0498] Preparation of 2-Trimethylsilylindole
[0499] Iodoaniline (2.19g, 10mmol), acylsilane (26g, 20mmol) prepared according to the procedure of Reference Example A), DABCO (1,4-diazabicyclo[2.2.2]octane, 3.36g, 30mmol) and Pd(OAC) 2 (112.25mg, 0.5mmol) in the mixture in 30ml DMF by N 2 / Vacuum degassed, then heated at 105°C for 36 hours. The mixture was cooled to room temperature, diluted with IPAc (isopropyl acetate, 100ml) and washed with 2 x 50ml of water. The IPAc layer was concentrated in vacuo and purified by silica gel chromatography to obtain 2-trimethylsilylindole and indole.
example 2-8
[0501] Following the procedure of Example 1, starting from appropriately substituted 2-iodoanilines and appropriate acylsilanes, the following compounds were prepared: where R 17 It is a hydroxyl protecting group that can be removed under mild acid hydrolysis conditions.
example 9
[0503] Preparation of indole
[0504] A mixture of the 2-trimethylsilylindole product of Example 1 in 5 mL of methanol was treated with 2.5N HCl (2.11 mL, 5.2 mmol) and the reaction mixture was allowed to mature at room temperature for 2 hours. Additional isopropyl acetate (50ml) and water (10ml) were added. The layers were separated and the organic layer was concentrated in vacuo. The residual oil was chromatographed on silica gel to give indole as a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com