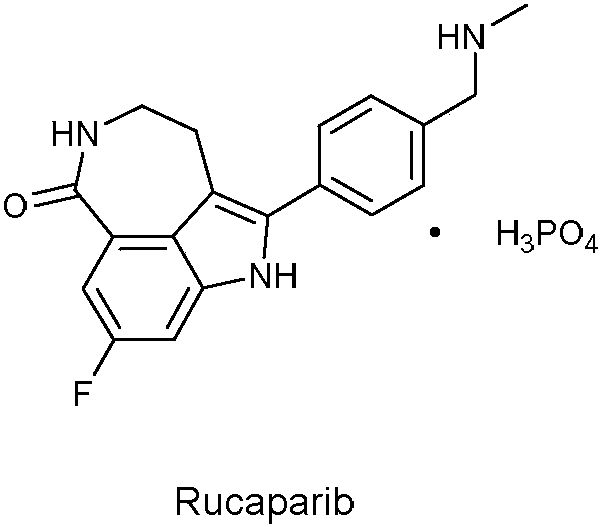
A kind of preparation method of rucaparib key intermediate
An intermediate and key technology, applied in the field of drug synthesis, can solve the problems of low yield of catalytic hydrogenation reaction, unfavorable industrial production, severe heat release, etc., and achieve easy and effective control of reaction conditions, reasonable route design, and cheap and easy-to-obtain raw materials Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
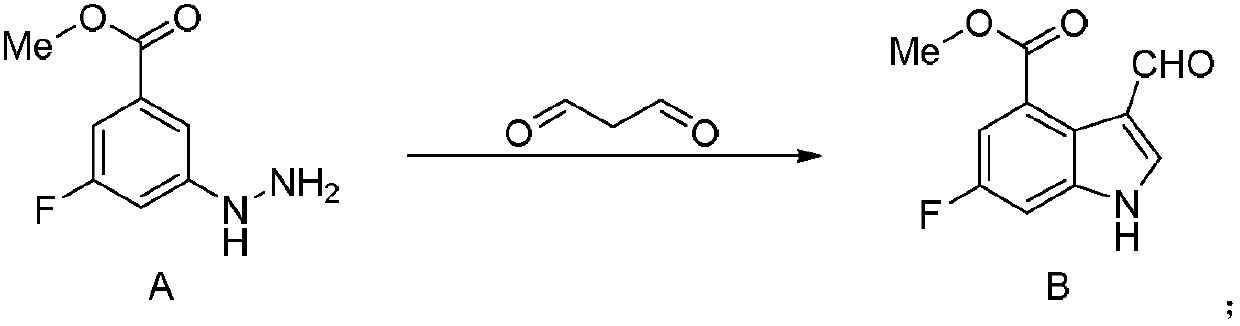
[0026] (1) Synthesis of 6-fluoro-3-formyl-1H-indole-4-carboxylic acid methyl ester (compound B):
[0027] Add 3-fluoro-5-hydrazino-benzoic acid methyl ester (9.20g, 0.05mol) and malondialdehyde (6.49g, 0.09mol) into 50mL water, add 90% formic acid (1.15g, 0.025mol), then add Sodium hydrogen phosphate (21.30g, 0.15mol), the reaction mixture was reacted at 40°C for 5 hours, TLC was spotted to confirm the completion of the reaction, water (200mL) and ethyl acetate (200mL x 3) were added for separation and extraction, and the organic layer was separated and collected , the organic layer was washed with saturated aqueous sodium chloride solution (100 mL), and the organic phase was collected and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure to obtain 9.36g white solid 6-fluoro-3-formyl-1H-indole-4-carboxylic acid methyl ester (compound B), the yield was 84.6%, and the purity was 98.3% (HPLC area normalization method ) The reaction formula...
Embodiment 2
[0032] The preparation method is the same as in Example 1, but the molar ratio between compound A, malondialdehyde, catalyst and buffering agent in step (1) is 1.0: 1.5: 0.1: 2.0, the reaction temperature is 80 ° C, and the reaction time is 2 hours 8.27g of methyl 6-fluoro-3-formyl-1H-indole-4-carboxylate (compound B) was obtained as a white solid, with a yield of 74.8% and a purity of 94.4% (HPLC area normalization method).
Embodiment 3
[0034] The preparation method is the same as in Example 1, but the molar ratio between compound A, malondialdehyde, catalyst and buffering agent in step (1) is 1.0: 2.5: 0.6: 4.0, the reaction temperature is 20 ° C, and the reaction time is 8 hours 7.28g of methyl 6-fluoro-3-formyl-1H-indole-4-carboxylate (compound B) was obtained as a white solid, with a yield of 65.8% and a purity of 93.5% (HPLC area normalization method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com