Preparation method of K252c derivative and application of K252c derivative in preparation of anti-tumor metastasis medicine
A technology of derivatives and drugs, applied in the field of medicine, can solve the problems of not finding the relationship between ROCK2 inhibitors and anti-tumor metastasis, and the anti-tumor metastasis effect is not ideal, achieving significant anti-tumor metastasis effect, easy operation and implementation, synthesis Simple route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
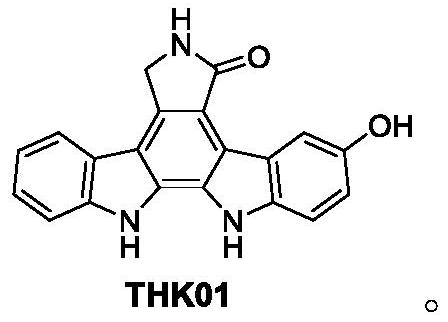
[0052] The total synthesis of embodiment 1.THK01
[0053]
[0054]Weigh 10g (66mmol) of o-nitrobenzaldehyde (a, 2-nitro-benzaldehyde) and 21g (66mmol) of Phosphorus ylides into a 250mL reaction flask, add 50mL of anhydrous acetonitrile, and stir at reflux for 12h at 60°C . The progress of the reaction was detected by TLC until the disappearance of the raw materials, and the reaction product was concentrated under reduced pressure and separated by silica gel column chromatography (mobile phase was dichloromethane) to obtain light yellow solid b (12.6 g, 100%).
[0055]
[0056] Weigh 12.6g (66mmol) of product b into a three-necked reaction flask, and add 40mL of anhydrous dichloromethane to dissolve it. Connect the device so that the reaction system is filled with N 2 , add 26.7g (264mmol) triethylamine and 34.9g (132mmol) tert-butyldimethylsilyl trifluoromethanesulfonate (TBDMSOTf) dropwise successively, stir and reflux at 50°C for 4h, add triethylamine (6.7 g, 66mmol...
Embodiment 2
[0067] Example 2. Synthesis of Derivatives
[0068] According to the above synthetic route, the derivatives of THK01 are obtained by replacing the reaction substrate of Step1 or Step5, and the reaction conditions and purification methods are the same. The specific route can refer to the synthetic route.
[0069] THK04, THK05: THK04 and THK05 were obtained by reduction reaction of THK02, and the reduction products were prepared by HPLC high-pressure liquid phase (35% acetonitrile water, Agilent Pursuit C18 column, 10 μm, 21.2×250mm, which were used in the following unless otherwise specified) preparative column) to obtain white solid THK04 (t R =54min, productive rate 45%) and THK05 (t R =70 min, yield 44%).
[0070] THK06, THK07, THK08: Replace the substrate in the first step with o-nitro-m-methoxybenzaldehyde, and the rest of the operations are the same as for the synthesis of THK01. Separate through silica gel column chromatography to obtain THK06 (the 5th step reaction,...
Embodiment 3
[0079] Example 3. Identification of Compounds
[0080] THK01: white solid. HRESIMS gives the quasi-molecular ion peak as [M+H] + m / z328.1080 (calculated value is 328.1086), molecular formula is C 20 h 14 N 3 o 2 . 1 H NMR (600MHz, DMSO-d 6):δ11.78(1H,s),11.29(1H,s),8.96(1H,s),8.62(1H,d,J=2.5Hz),8.44(1H,s),8.01(1H,d, J=7.7Hz),7.74(1H,d,J=8.1Hz),7.49(1H,d,J=8.6Hz),7.45(1H,t,J=8.1Hz),7.28(1H,t,J=8.1Hz) 7.7Hz), 6.91(1H, dd, J=2.6, 8.6Hz), 4.93(2H, s). The spectrum data of the synthesized compound THK01 is consistent with the natural product obtained from the previous separation and extraction (Bioactive indolocarbazoles from the marine-derived Streptomycessp .DT-A61[J].J.Nat.Prod.2018,81,949-956.).
[0081] THK02: yellow solid. HRESIMS gives the quasi-molecular ion peak as [M+Na] + m / z 378.0848 (calculated value is 378.0855), molecular formula is C 21 h 13 N 3 o 3 . 1 H NMR (600MHz, DMSO-d 6 ): δ11.80(1H,s),11.63(1H,s),10.97(1H,s),8.99(1H,d,J=7.9Hz),8.58(1H,d,J=2....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com