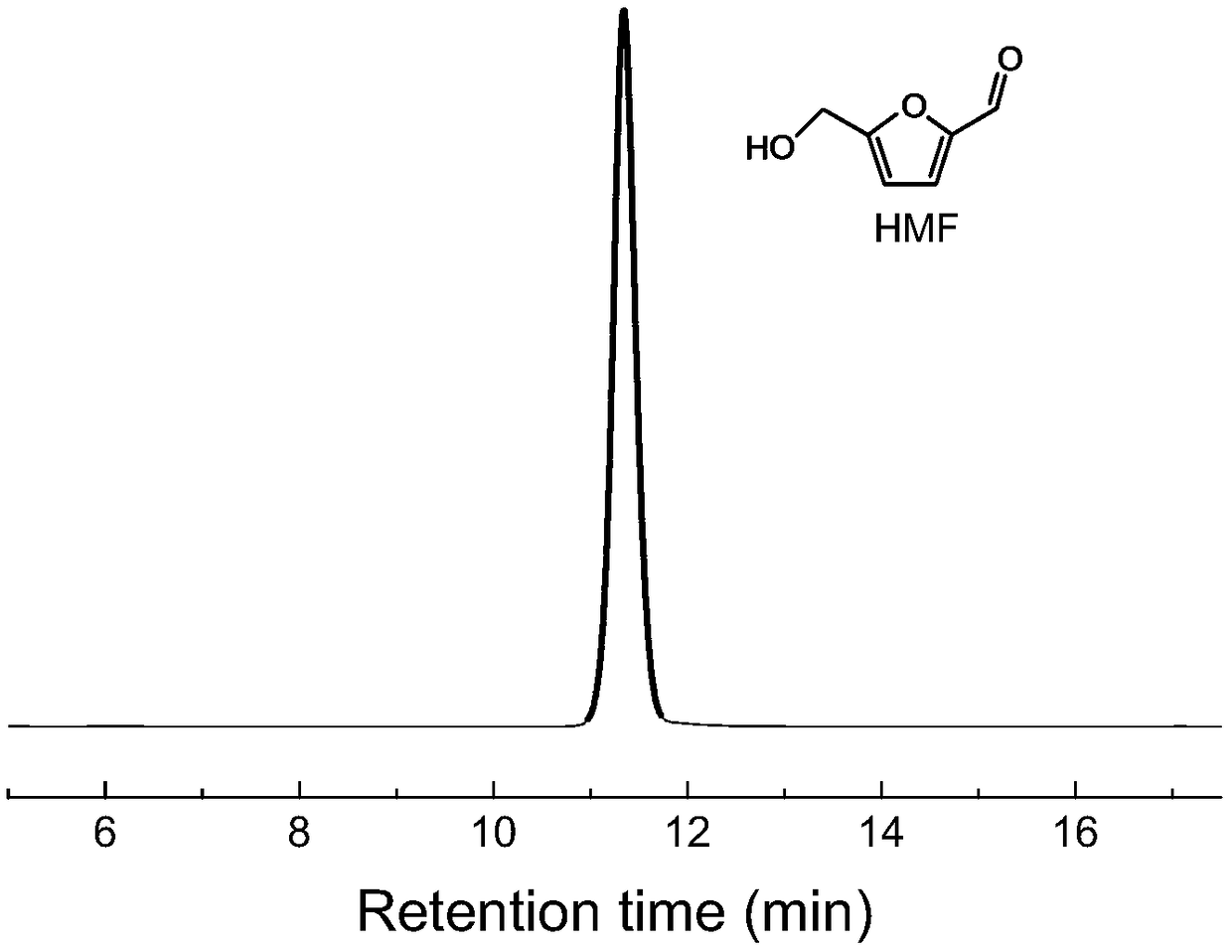
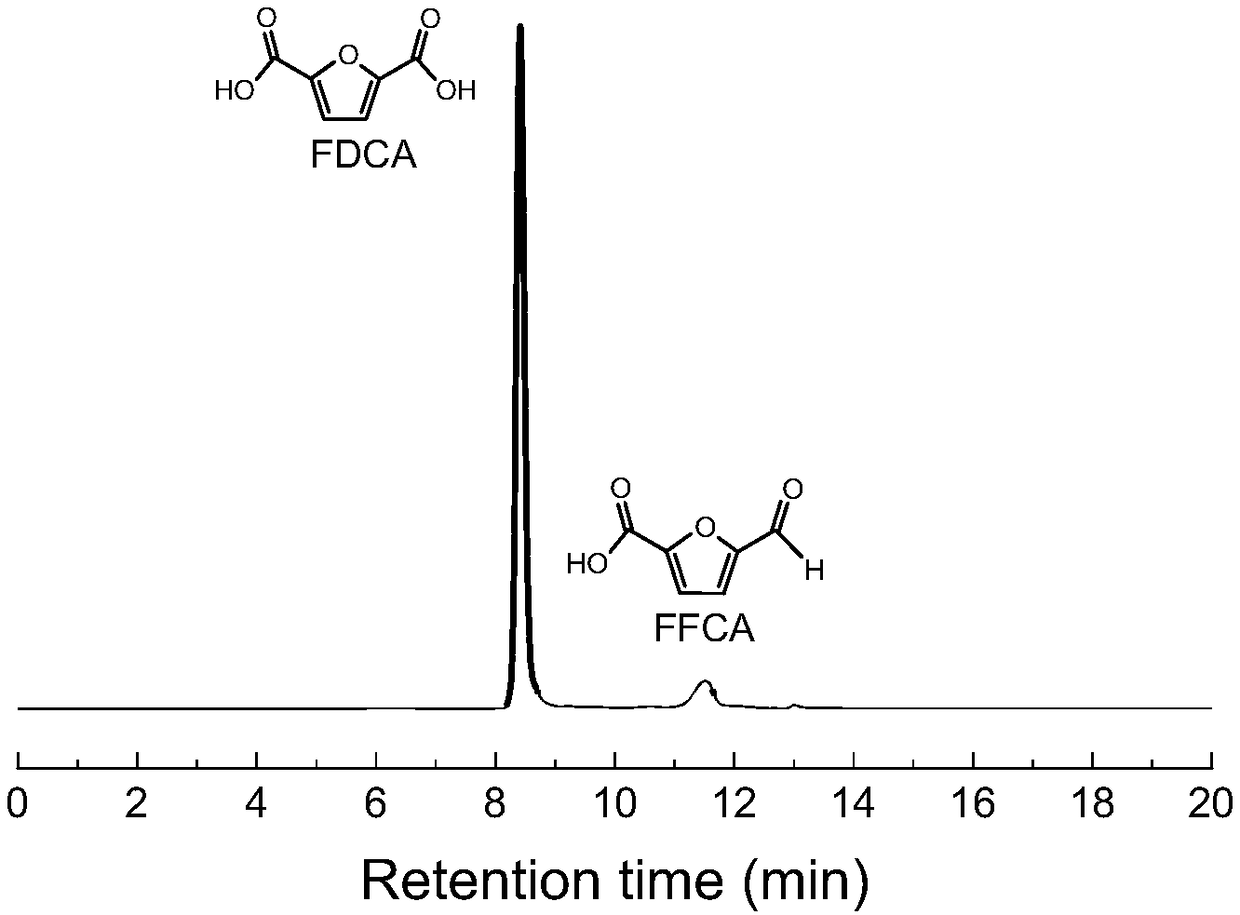
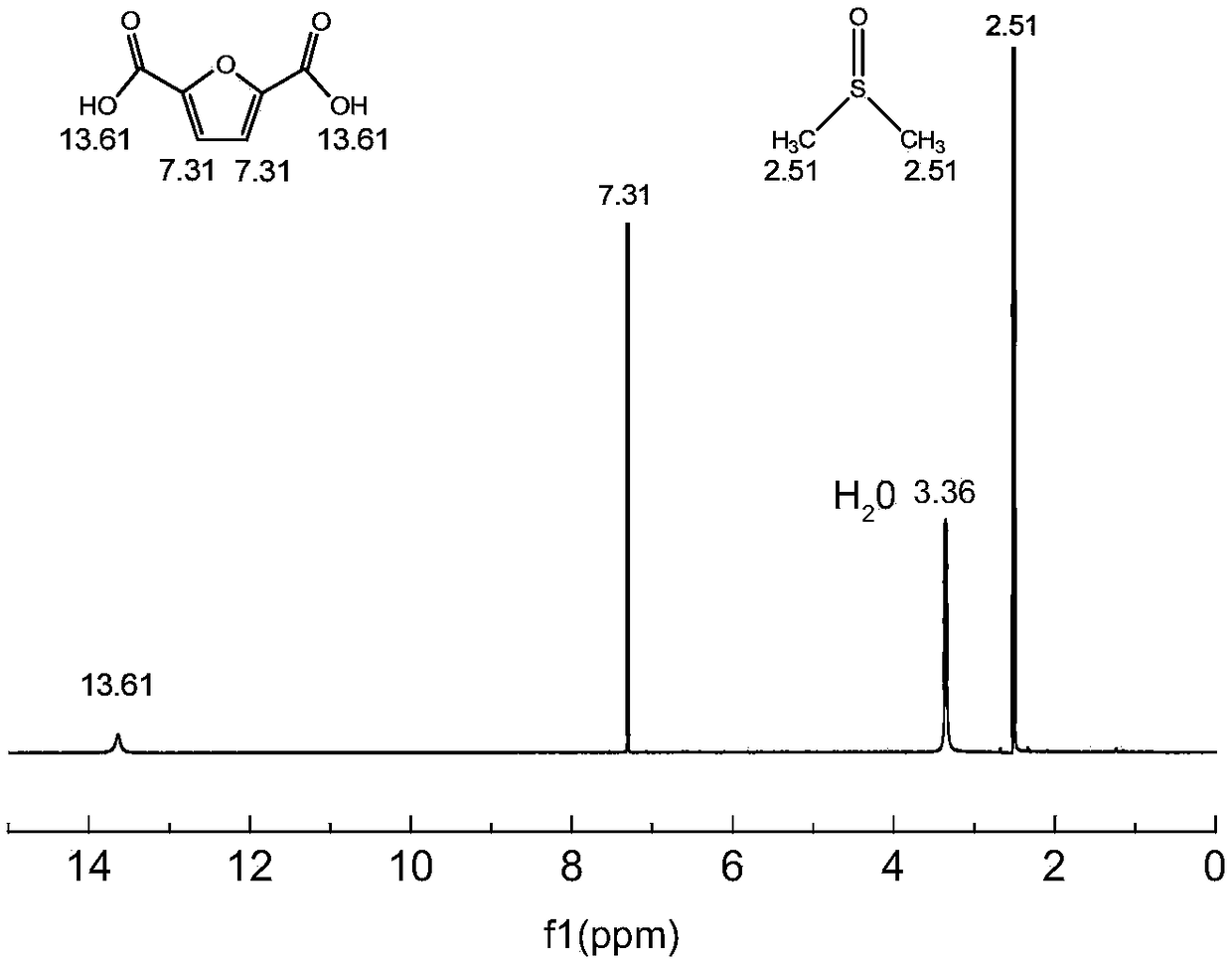
Method and system for continuously synthesizing furandicarboxylic acid
A technology of furandicarboxylic acid and solid acid, which is applied in the direction of organic chemistry, can solve the problem of low yield of target products, achieve the effects of inhibiting side reactions, shortening reaction time, and promoting oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-4
[0048] Preparation of HMF by Dehydration of Sugars
[0049] Add 4g fructose, 16g DMSO and 1g Amberlyst-15 catalyst into a 50mL three-necked flask, pass N 2 Evacuate, heat up to the temperature shown in Table 1, fully stir, and react for 90 minutes to obtain HMF solution. The HMF content in the product liquid was analyzed by high performance liquid chromatography to obtain the HMF yield, as shown in Table 1.
[0050] The productive rate of fructose dehydration preparation HMF under table 1 different temperatures
[0051]
[0052] Wherein, the calculation method of HMF productive rate is as follows:
[0053]
Embodiment 5-7
[0055] Other process conditions and experimental steps are the same as in Example 4, but different reaction times are used in the stage of fructose dehydration reaction to prepare HMF. The reaction conditions and results are shown in Table 2.
[0056] The productive rate of fructose dehydration preparation HMF under table 2 different reaction times
[0057]
[0058]
[0059] It can be seen from Table 2 that in the early stage, as the reaction time increases, the yield of HMF basically shows an increasing trend. When the reaction time reaches 90 minutes, the yield of HMF reaches the maximum value. As the reaction time continues to increase, side reactions occur in HMF, and the yield begins reduce.
Embodiment 8-9
[0061] Other process conditions and experimental steps are the same as in Example 4, but different solvents are used in the stage of fructose dehydration reaction to prepare HMF, and the reaction results are shown in Table 3 below.
[0062] The productive rate that fructose dehydration prepares HMF in the different solvents of table 3
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com