Fluid hemostatic adhesive as well as preparation method and application thereof
A technology for fluid and glue products, applied in the field of fluid hemostatic glue and its preparation, can solve problems such as tissue irritation and influence on wound healing, enhance the speed of gel formation and gel strength, improve wound closure and hemostatic effects, and is easy to operate Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
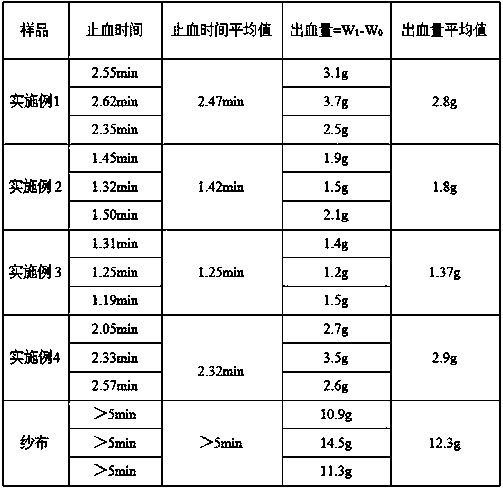
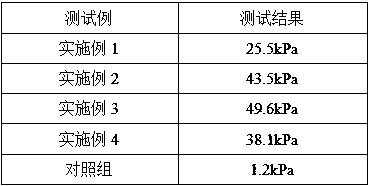
Examples
Embodiment 1
[0035] A fluid hemostatic glue, comprising a hemostatic effective component and a fluid matrix, the hemostatic effective component includes component A, component B and component C, wherein component A is 0.5g gelatin, and component B is 0.5g α-glycerin Sodium Phosphate, Component C is 0.5g polylysine, the fluid base consists of 10g saline and 0.5mg glycerin. Wherein the jelly strength of gelatin is 200 Bloom / g, and the source of gelatin is pig source.
[0036] The preparation method of the fluid hemostatic gel is as follows: Weigh 0.5g gelatin, 0.5g α-sodium glycerophosphate and 0.5g polylysine in a container, put in an ice bath, keep the temperature at 2°C, add 10g normal saline, and weigh another Add 0.5mg of glycerin into a container, stir to make it evenly mixed, take it out, put it into a container, and irradiate to obtain a fluid hemostatic gel product, which is stored at low temperature for future use.
Embodiment 2
[0038] A fluid hemostatic glue, comprising a hemostatic effective component and a fluid matrix, the hemostatic effective component includes component A, component B and component C, wherein component A is 0.5g gelatin, and component B is 0.5g α-glycerin Sodium Phosphate, Component C is 0.5g polylysine, the fluid base consists of 10g saline and 0.5mg glycerol. Wherein the jelly strength of gelatin is 200 Bloom / g, and the source of gelatin is pig source.
[0039]Wherein the gelatin of component A is modified gelatin, and the preparation method of the modified gelatin is: weigh 1g of gelatin, dissolve it in 20ml of deionized water, add 0.0125g of EDC and NHS, stir and react at 30°C for 3h, Add ethanol to soak and wash, repeat three times, pre-freeze at -80°C, and freeze-dry using a low-temperature and low-pressure freezer to obtain modified gelatin.
[0040] The preparation method of the fluid hemostatic gel is as follows: Weigh 0.5g of the above-mentioned modified gelatin, 0.5g...
Embodiment 3
[0042] A fluid hemostatic gel, comprising a hemostatic effective component and a fluid matrix, the hemostatic effective component includes component A, component B and component C, wherein component A is 1 g of gelatin, and component B is 0.6 g of β-glycerophosphate Sodium, Component C was 0.6 g polyethyleneimine, and the fluid base consisted of 11 g saline and 2.2 mg sodium lauryl sulfate. Wherein the jelly strength of gelatin is 300 Bloom / g, and the source of gelatin is pig source.
[0043] Wherein the component A gelatin is modified gelatin, the preparation method of the modified gelatin is: weigh 2g of gelatin, dissolve it in 20ml of deionized water, add 0.0250g of EDC and NHS, and stir and react at 25°C for 2.5h , add ethanol to soak and wash, repeat three times, pre-freeze at -80°C, and freeze-dry using a low-temperature and low-pressure freezer to obtain modified gelatin.
[0044] The preparation method of the fluid hemostatic gel is as follows: Weigh 1g of the above-m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com