Method for Determination of Dissolution Rate of 5 Components in Compound Reserpine Tablets by Ultra High Performance Liquid Chromatography
A technology of compound reserpine and ultra-high performance liquid phase, which is applied in the direction of measuring devices, material separation, and analysis of materials, can solve the problems of large sample volume and long running time of samples in pharmacopoeia methods, and achieve high sensitivity, good selectivity, The effect of the separation effect is obvious
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The method for measuring the dissolution rate of 5 components in compound reserpine tablets provided by the present embodiment with ultra-high performance liquid chromatography is as follows:
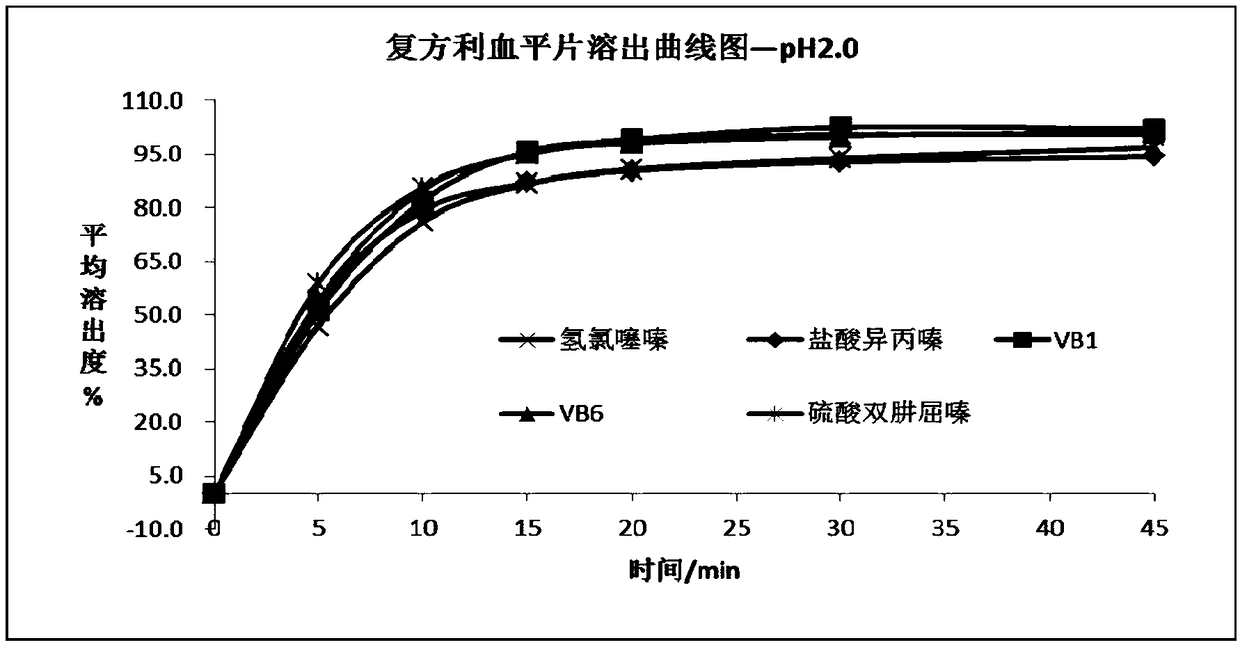
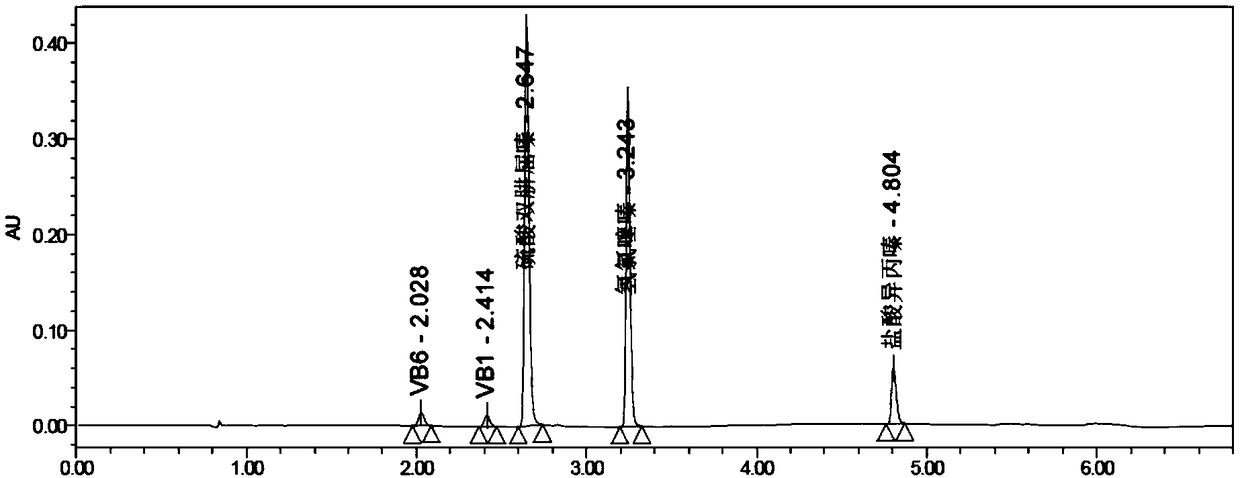
[0072] (1) Accurately weigh the appropriate amount of vitamin B1 reference substance, vitamin B6 reference substance, hydrochlorothiazide reference substance, promethazine hydrochloride reference substance, and dihydralazine sulfate reference substance, and finally add the corresponding dissolution medium to dilute to a concentration of vitamin B1 control substance 1 μg / mL of vitamin B6 reference substance, 1 μg / mL of vitamin B6 reference substance, 3.1 μg / mL of hydrochlorothiazide reference substance, 2.1 μg / mL of promethazine hydrochloride reference substance, and 4.2 μg / mL of dihydralazine sulfate reference substance, as a reference substance solution, detected by ultra-high performance liquid chromatography, the results are shown in Figure 20 , the results showed that the re...
Embodiment 2
[0094] The method for measuring the dissolution rate of five components in compound reserpine tablets by ultra-high performance liquid chromatography provided in this example is basically the same as that in Example 1, the difference is that the dissolution medium used in this example is pH4.5 Sodium acetate buffer solution.
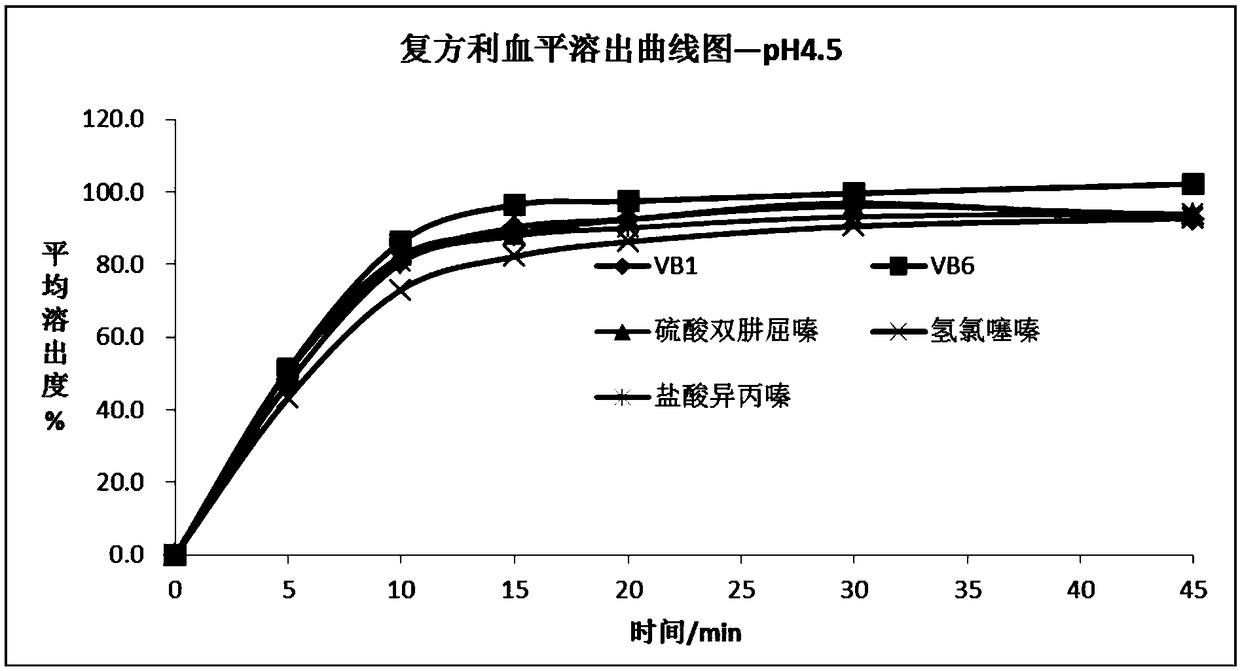
[0095] see results image 3 and Figure 4 .
[0096] In the dissolution medium of the sodium acetate buffer solution of pH4.5, the peak shape of each active component (vitamin B1, vitamin B6, hydrochlorothiazide, dihydralazine sulfate, promethazine hydrochloride) is good ( Figure 4 ), can accurately quantitatively analyze, and after 45min, the dissolution rate of each active component is more than 85% ( image 3 ).
Embodiment 3
[0098] The method provided in this example to measure the dissolution rate of five components in compound reserpine tablets by ultra-high performance liquid chromatography is basically the same as that in Example 1. The difference is that the dissolution medium used in this example is pH 6.8. Sodium phosphate buffer solution.
[0099] see results Figure 5 and Figure 6 .
[0100]In the dissolution medium of the sodium phosphate buffer solution of pH6.8, the peak shape of each active component (vitamin B1, vitamin B6, hydrochlorothiazide, dihydralazine sulfate, promethazine hydrochloride) is good ( Figure 6 ), can accurately quantitatively analyze, and after 45min, the dissolution rate of each active component is more than 85% ( Figure 5 ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com