Compound, preparation method thereof and application of compound in uranium analysis
A technology for compounds and detection methods, applied in chemical instruments and methods, analytical materials, material excitation analysis, etc., to achieve the effect of simple processing and simple implementation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
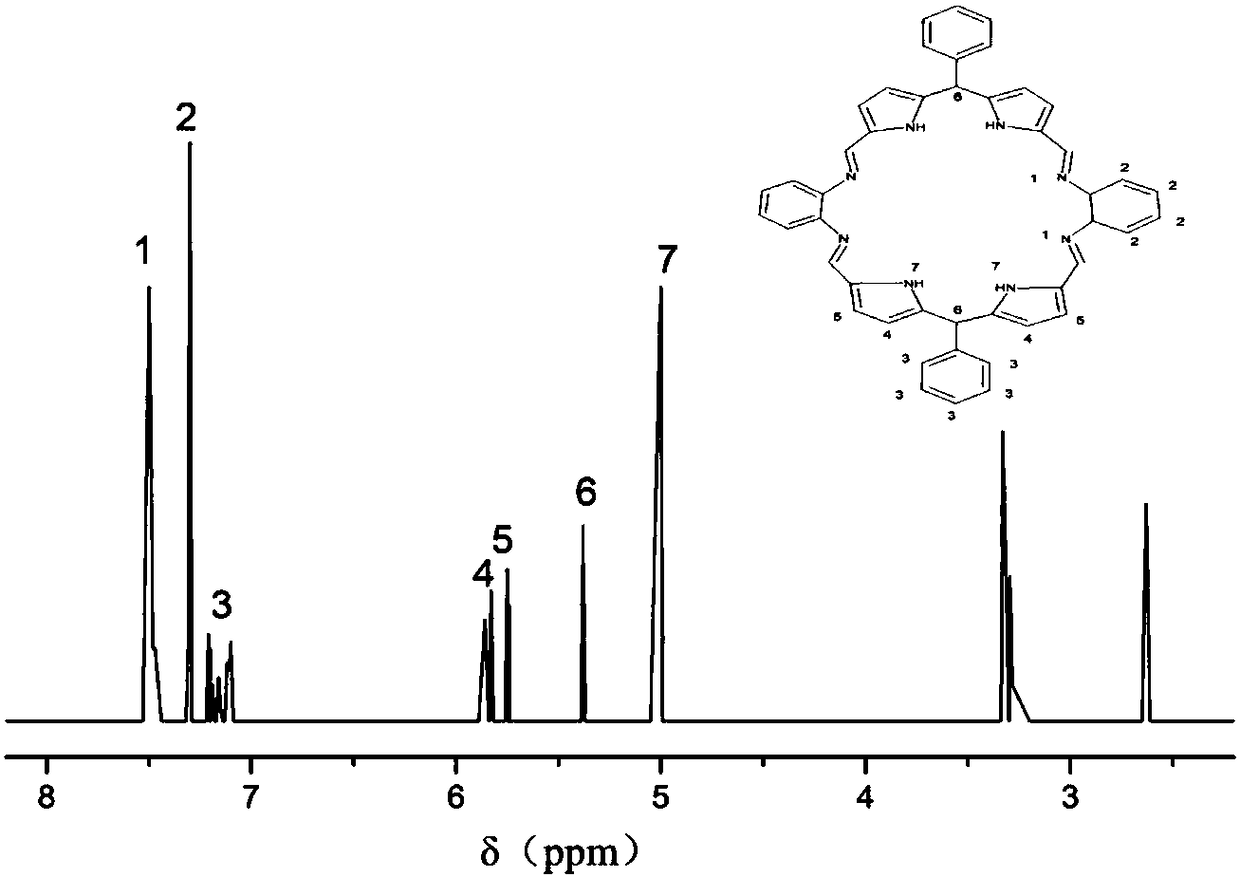
[0054] The present embodiment provides a compound (polydentate macrocyclic ligand H 4 L).
[0055] Its chemical structural formula is as follows:
[0056]
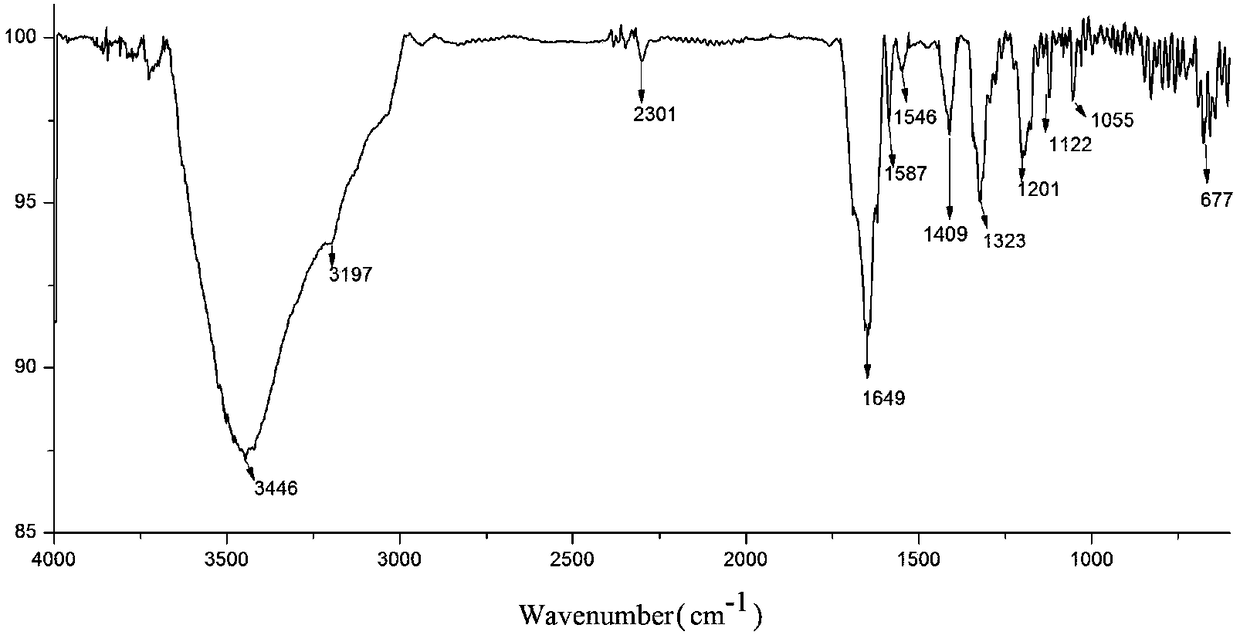
[0057] figure 1 Ligand H described in this example 4 The infrared spectrum of L, in figure 1 Among them, there are at 3346.71cm -1 Absorption attribution δ N-H The vibration of 3197cm -1 The left and right absorption peaks belong to the stretching vibration peaks of C-H on the pyrrole ring and benzene ring, and the compound is at 1649.57cm -1 There is a stretching vibration peak of benzene ring C=C, at 1587.48cm -1 The absorption at is attributed to the δ on the pyrrole ring C-H vibration at 1055cm -1 、677.45cm -1 The absorption bands appearing everywhere can be attributed to the meta-substitution absorption peak on the benzene ring.
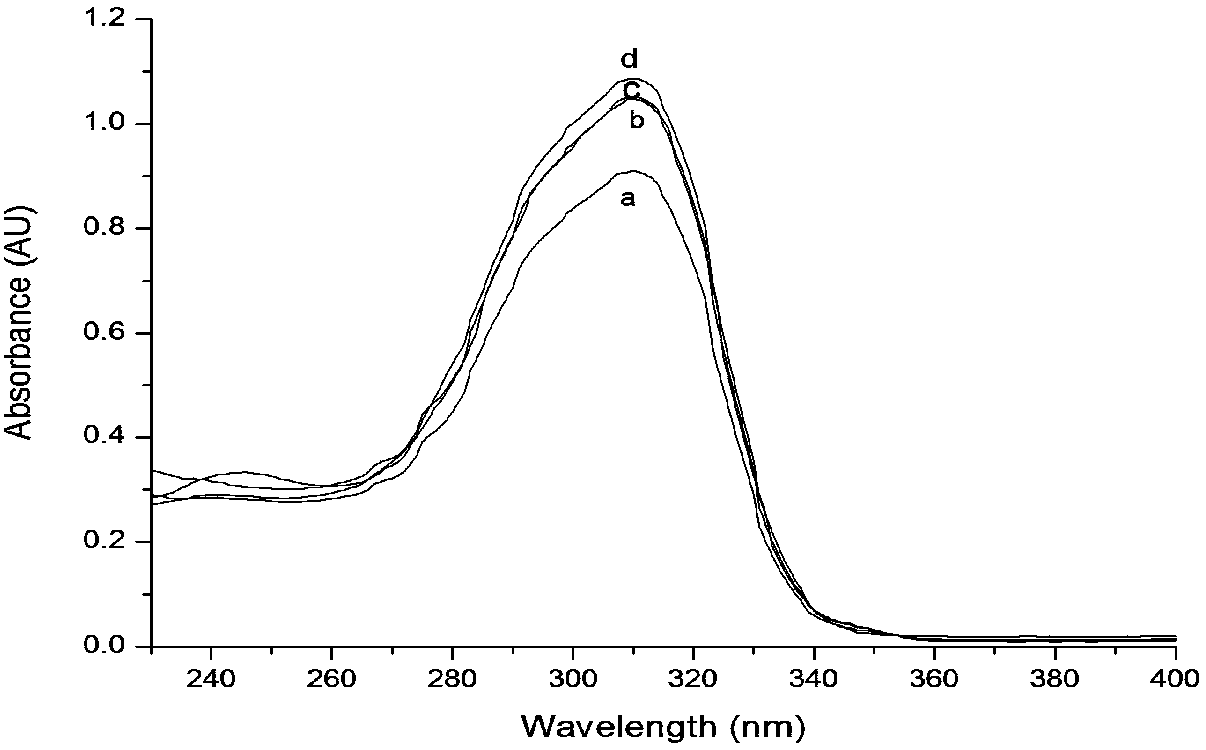
[0058] figure 2 Ligand H described in this example 4 UV spectra of L and uranyl complexes, in figure 2 Among them, there is a characteristic absorption peak on the benzene...
Embodiment 2
[0062] The present embodiment provides a compound (polydentate macrocyclic ligand H 4 L) and preparation method thereof, its preparation method is as follows:
[0063] Add 0.81 g of phenylenedipyrrole formaldehyde and 0.35 g of o-phenylenediamine into 100 ml of DCM / MeOH (DCM / MeOH=10:1) solution, drop 2 drops of TFA, the color gradually deepens and turns brown. The reaction solution was stirred overnight, the solvent was spin-dried under reduced pressure, methanol was added, ultrasonicated, filtered, washed with methanol, and dried to obtain the macrocyclic ligand (H 4 L).
[0064] Get the obtained macrocyclic ligand (H 4 L) Characterize according to the same conditions as in Example 1, and its characterization information is the same as in Example 1.
Embodiment 3
[0066] This embodiment provides a multidentate macrocyclic ligand H 4 L and its preparation method, the preparation method is as follows: 0.8g of phenylenedipyrrole formaldehyde and 0.43g of o-phenylenediamine are added to 100ml of DCM / MeOH (DCM / MeOH=10:1.5) solution, 2 drops of TFA are added dropwise, The color gradually deepens to brown. The reaction solution was stirred overnight, the solvent was spin-dried under reduced pressure, methanol was added, ultrasonicated, filtered, washed with methanol, and dried to obtain the macrocyclic ligand (H 4 L).
[0067] Get the obtained macrocyclic ligand (H 4 L) Characterize according to the same conditions as in Example 1, and its characterization information is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com