Alpha-aryl substituted amino acid derivative preparation method
An amino acid and derivative technology, applied in the field of compound preparation, achieves the effects of mild reaction conditions, simple reaction and post-processing purification process, high selectivity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
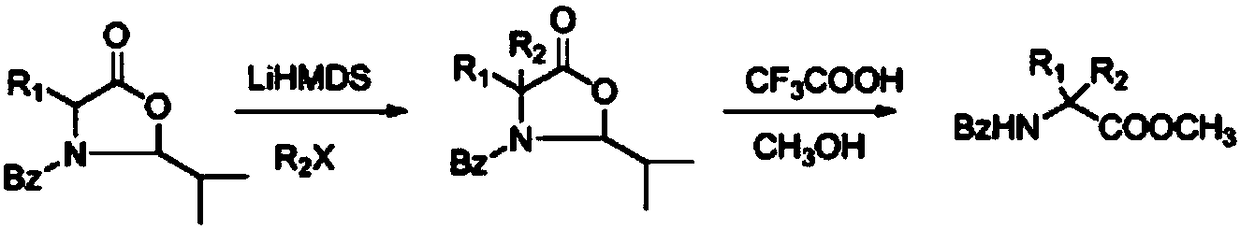
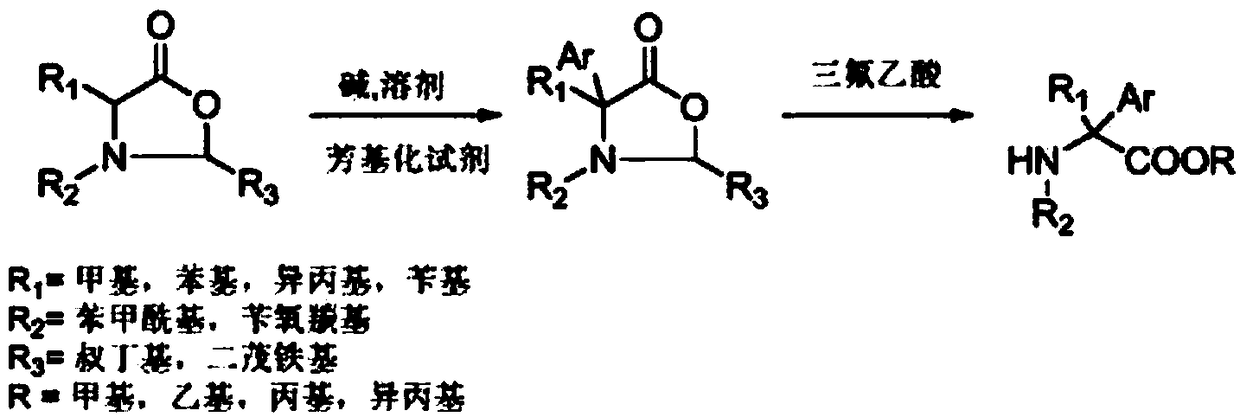
[0020] Under an inert gas atmosphere, 0.261 grams (1 mmol) of oxazolidinone compounds (wherein R1 = methyl, R2 = benzoyl, R3 = tert-butyl) were dissolved in 10 ml of tetrahydrofuran at -78 ° C, and 0.183 g (1.1 mmol) lithium hexamethylsilylamide, stirred and reacted for half an hour, then added 0.172 g (1.1 mmol) bromobenzene, reacted at -78°C for 2 hours, then slowly warmed up to room temperature, and reacted for 12 hours. 5 mL of saturated ammonium chloride solution was added, the mixture was extracted with ethyl acetate (3×10 mL), the combined extracts were dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the crude product was dissolved in 10 ml of 2M trifluoroacetic acid methanol solution, sealed and heated at 130°C for 48 hours. After cooling to room temperature, the solvent was evaporated under reduced pressure, 50 ml of dichloromethane was added, and the organic phase was washed with water (3×10 ml) and dried over anhydrous ...
Embodiment 2
[0022] Under an inert atmosphere, 0.337 grams (1 mmol) of oxazolidinone compounds (wherein R1 = benzyl, R2 = benzoyl, R3 = tert-butyl) were dissolved in 10 ml of toluene at -78 ° C, and 0.219 g (1.1mmol) potassium hexamethylsilylamide, stirred for half an hour, then added 0.249 grams (1.1mmol) of phenyl trifluoromethanesulfonate, reacted at -78°C for 2 hours, then slowly warmed up to room temperature, reaction 12 Hour. 5 mL of saturated ammonium chloride solution was added, the mixture was extracted with ethyl acetate (3×10 mL), the combined extracts were dried over anhydrous sodium sulfate. The solvent was evaporated under reduced pressure, and the crude product was dissolved in 10 ml of 2M ethanol trifluoroacetic acid solution, sealed and heated at 130°C for 48 hours. After cooling to room temperature, the solvent was evaporated under reduced pressure, 50 ml of dichloromethane was added, and the organic phase was washed with water (3×10 ml) and dried over anhydrous sodium s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com