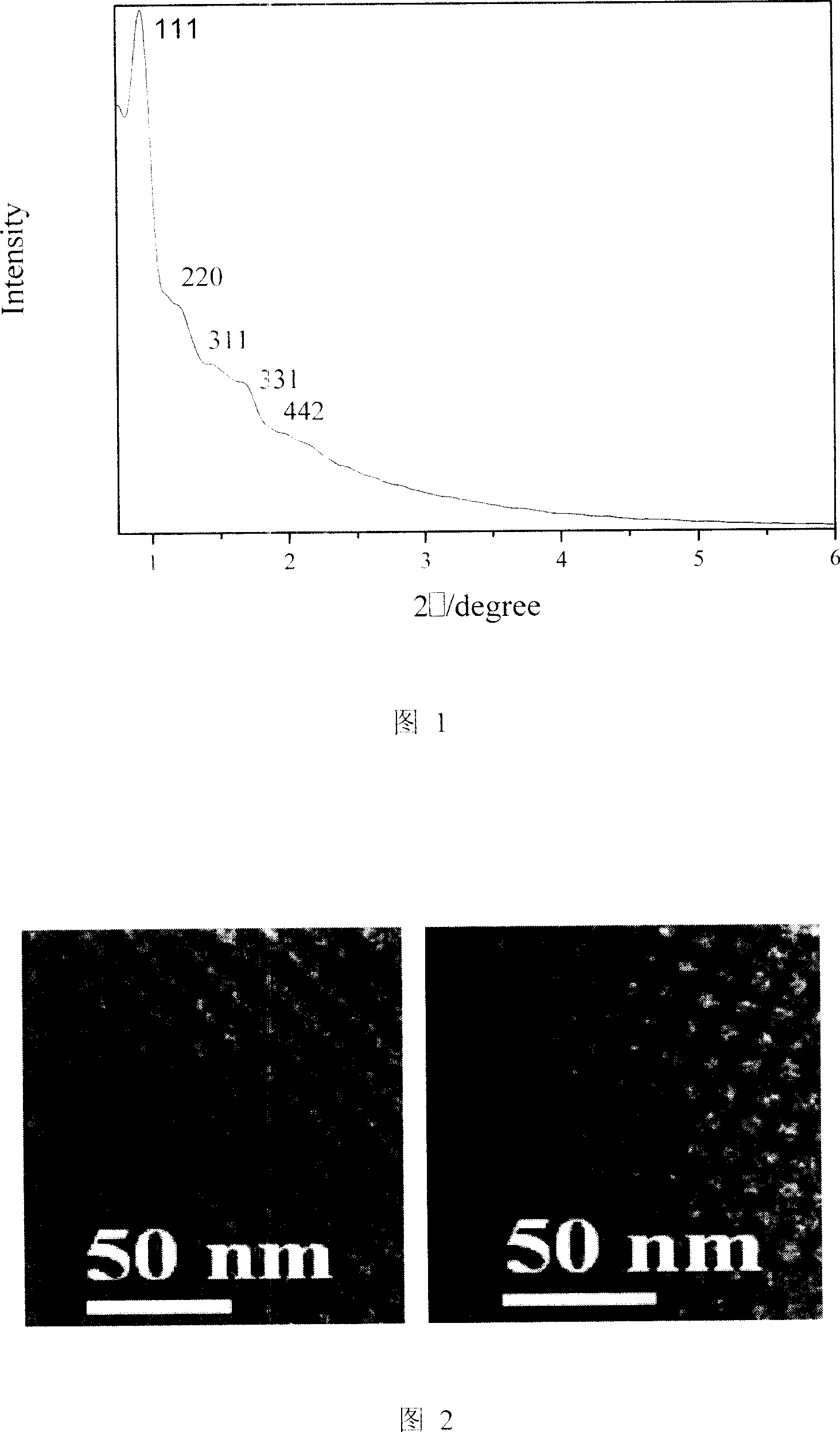
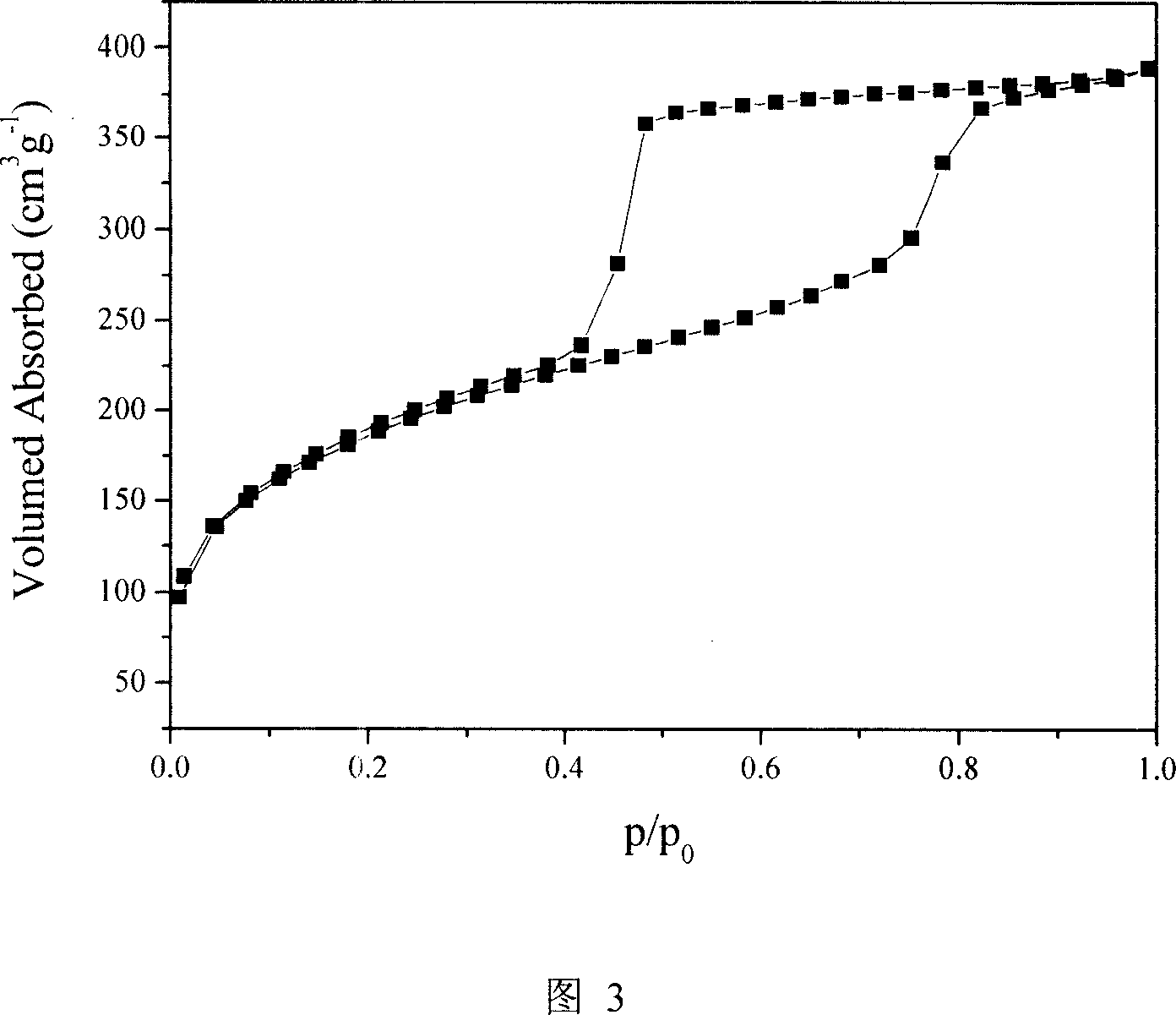
Heterogenous organic metal catalyst with three-dimensional mesoporous structure, preparation method and application thereof
An organometallic, three-dimensional mesoporous technology, applied in the direction of isomerization preparation, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem that the activity and selectivity remain unchanged and the catalyst cannot be recycled Utilization, heavy metal ion pollution and other problems, to achieve the effect of high selectivity and acquisition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1: At 30°C, mix 0.5g F127, 30g 2M hydrochloric acid, 2.5g and 0.69mL 1,3,5-trimethylbenzene, stir at 30°C until all surfactants are dissolved, then add 2.0mL TEOS and continue stirring 1h, 0.41mL of 2-(diphenylsulfone)ethyltriethoxysilane was added, and stirring was continued at the same temperature for 24 hours. The obtained mixed solution was transferred to a polytetrafluoroethylene hydrothermal bottle, and hydrothermally reacted in an oven at 100°C for 1 day. After cooling, a white powder was obtained after suction filtration, washing, and natural drying at room temperature.
[0031] Step 2: Put 0.5g of the sample without extracting surfactant and 30g of 2M hydrochloric acid into a polytetrafluoroethylene water thermos bottle, and age them at 100°C for 48 hours, then filter, wash and dry them, and finally extract the samples The obtained white powder was extracted to remove the organic structure directing agent.
[0032] Step 3: Dissolve 95.86 mg RuCl at room t...
Embodiment 2
[0036] Step 1: Same as Step 1 in Example 1.
[0037] Step 2: Put 0.5g of the sample without extracting surfactant and 30g of 2M hydrochloric acid into a polytetrafluoroethylene water thermos bottle, age them at 120°C for 48 hours, filter them with suction, wash them, and dry them. The obtained white powder was extracted to remove the organic structure directing agent.
[0038] Step 3: Same as Step 3 in Example 1.
[0039] Step 4: with step 4 in embodiment 1, obtain the immobilized organometallic catalyst (Ru-PPh 2 - FDU-12-2) Catalyst.
[0040] Step 5: Same as Step 5 in Example 1.
Embodiment 3
[0042] Step 1: Same as Step 1 in Example 1.
[0043] Step 2: Put 0.5g of the sample without extracting surfactant and 30g of 2M hydrochloric acid into a polytetrafluoroethylene water thermos bottle, age them at 130°C for 48 hours, filter them with suction, wash them, and dry them. The obtained white powder was extracted to remove the organic structure directing agent.
[0044] Step 3: Same as Step 3 in Example 1.
[0045] Step 4: with step 4 in embodiment 1, obtain the immobilized organometallic catalyst (Ru-PPh 2 - FDU-12-3) Catalyst.
[0046] Step 5: Same as Step 5 in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com