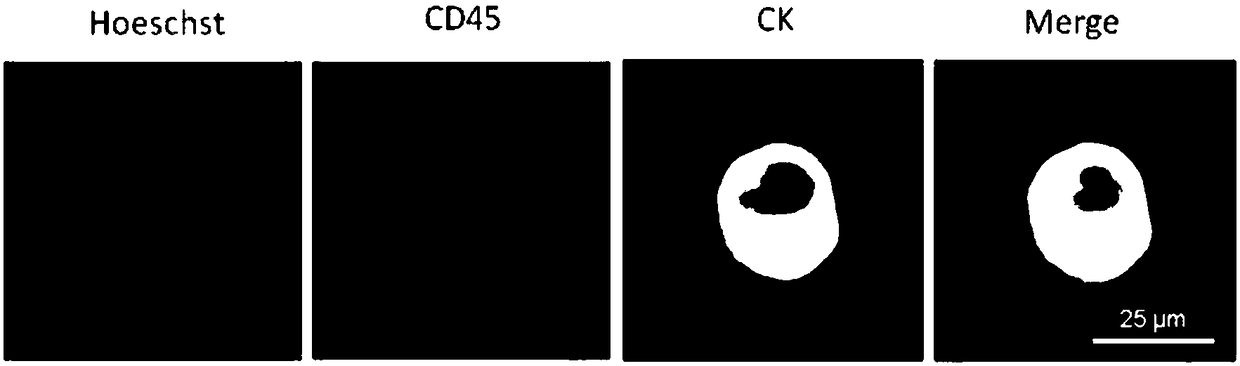
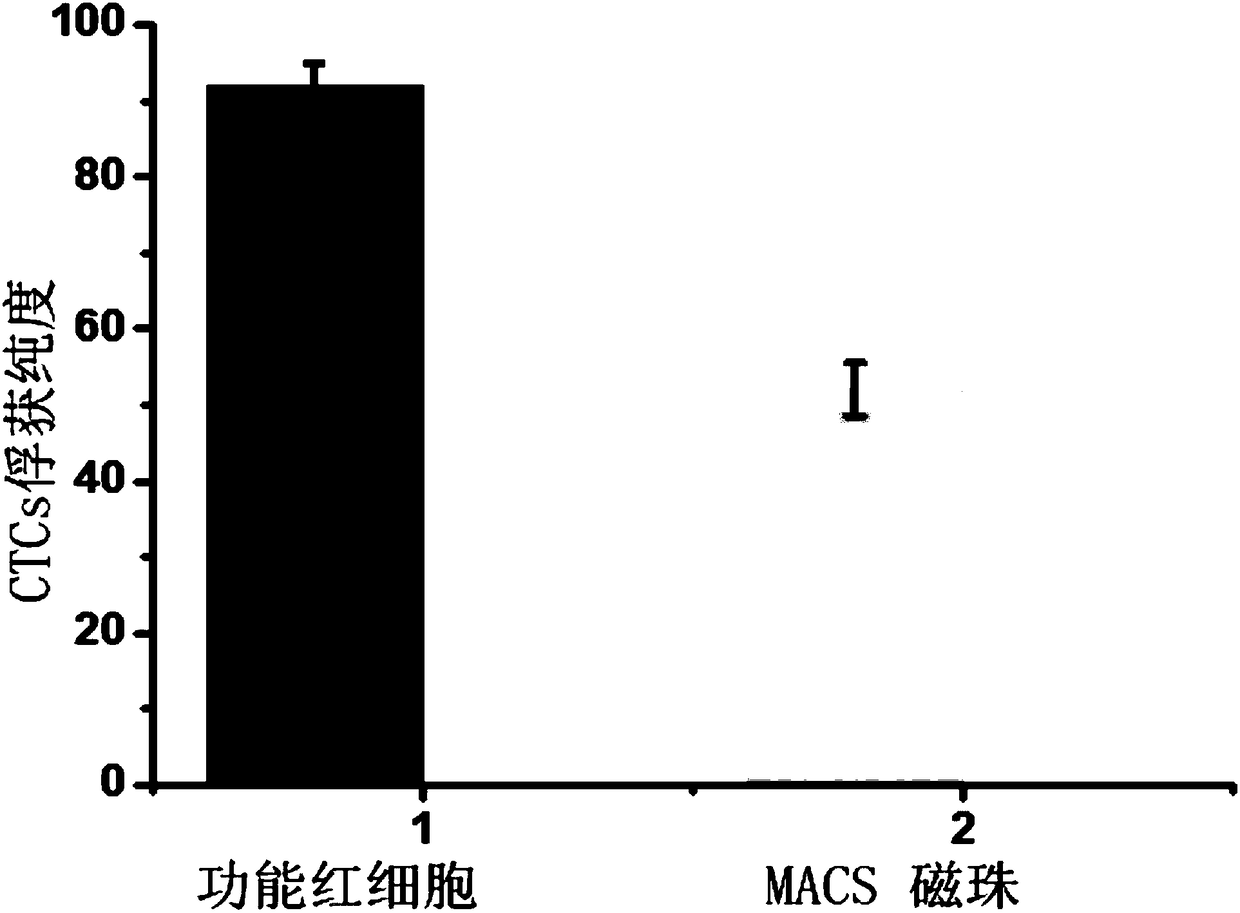
Functional red blood cell targeting circulating tumor cells (CTCs)
A technology for tumor cells and red blood cells, applied in the field of functional red blood cells, can solve the problems of low separation purity, difficulty in in-depth analysis, and difficulty in the purity of CTCs samples, and achieve the effect of avoiding interference and stable structure.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Erythrocyte modification of folic acid (FA) for specific recognition, capture and activity regulation of CTCs in vitro
[0037] (1) Erythrocyte modified folic acid (FA): first extract 100 μL erythrocytes from 1 mL of normal human blood, and then incubate with 50 μL (1 mg / mL) DSPE-PEG-FA (purchased from American Nanocs Company) (purchased from US nanocs company) for 30 minutes, and all samples were prepared at 1000 g After centrifugation for 10 minutes, they were washed three times with PBS before use to form FA-modified functional erythrocytes.
[0038] (2) In order to confirm that DSPE was effectively embedded in the red blood cell membrane, DSPE-PEG-Cy5 was used instead of DSPE-PEG-FA. First extract 100 μL red blood cells from 1 mL of normal human blood, then incubate with 50 μL (1 mg / mL) DSPE-PEG-Cy5 for 30 minutes, centrifuge all samples at 1000 g for 10 minutes, wash with PBS three times before use, and form Cy5-modified red blood cells . DSPE-PEG-Cy5 m...
Embodiment 2
[0044] Example 2 Red blood cell modified epithelial cell adhesion molecule antibody (RBC-antiEpCAM)
[0045] (1) Modification of biotin (Biotin) on the surface of red blood cells (RBC): 500 μL of red blood cells were suspended in 4 ml of PBS (pH7.4), and then 0.5 mg of DSPE-PEG-Biotin (purchased from American nanocs company) (3700D, 100 μg / mL) was added ), incubated at 37°C for 30 minutes with continuous stirring, DSPE was embedded in the phospholipid bilayer of RBC to obtain RBC-Biotin. The obtained RBC-Biotin was washed twice (PBS, pH 7.4, 400g, 5min),
[0046] (2) Modification of avidin (Avidin) on RBC-Biotin: under constant stirring, add 1 mg of Avidin solution (6.30×104 molecules / red blood cells) to the RBC-Biotin solution, and react at 4°C for 60 minutes . Formation of red cell RBC-Biotin-Avidin. The obtained RBC-Biotin-Avidin solution was washed twice (PBS, pH 7.4, 400 g, 5 minutes) to remove unbound avidin.
[0047] (3) Modification of epithelial cell adhesion mole...
Embodiment 3
[0053] Example 3 Red blood cell modified sialylated Lewis oligosaccharide-X antibody (RBC-anti-Sialyl Lewis X)
[0054] (1) Modification of biotin on the surface of red blood cells (RBC): 500 μL of red blood cells were suspended in 4 ml of PBS (pH 7.4), and then 0.5 mg of DSPE-PEG-Biotin (purchased from American nanocs company) (3700D, 100 μg / mL) was added ), incubated at 37°C for 30 minutes with continuous stirring, DSPE was embedded in the phospholipid bilayer of RBC to obtain RBC-Biotin. The obtained RBC-Biotin was washed twice (PBS, pH 7.4, 400g, 5min),
[0055] (2) Modification of avidin (Avidin) on RBC-Biotin: under constant stirring, add 1 mg of Avidin solution (6.30×104 molecules / red blood cells) to the RBC-Biotin solution, and react at 4°C for 60 minutes . Formation of red cell RBC-Biotin-Avidin. The obtained RBC-Biotin-Avidin solution was washed twice (PBS, pH 7.4, 400 g, 5 minutes) to remove unbound avidin.
[0056] (3) Modification of epithelial cell adhesion m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com