Multiple PCR primer group for detecting five fimbrial genes of enterotoxigenic escherichia coli, kit and detection method
A detection kit, Escherichia coli technology, applied in biochemical equipment and methods, recombinant DNA technology, and microbial determination/inspection, etc., can solve the problems of glass plate agglutination test limitations, etc. highly specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
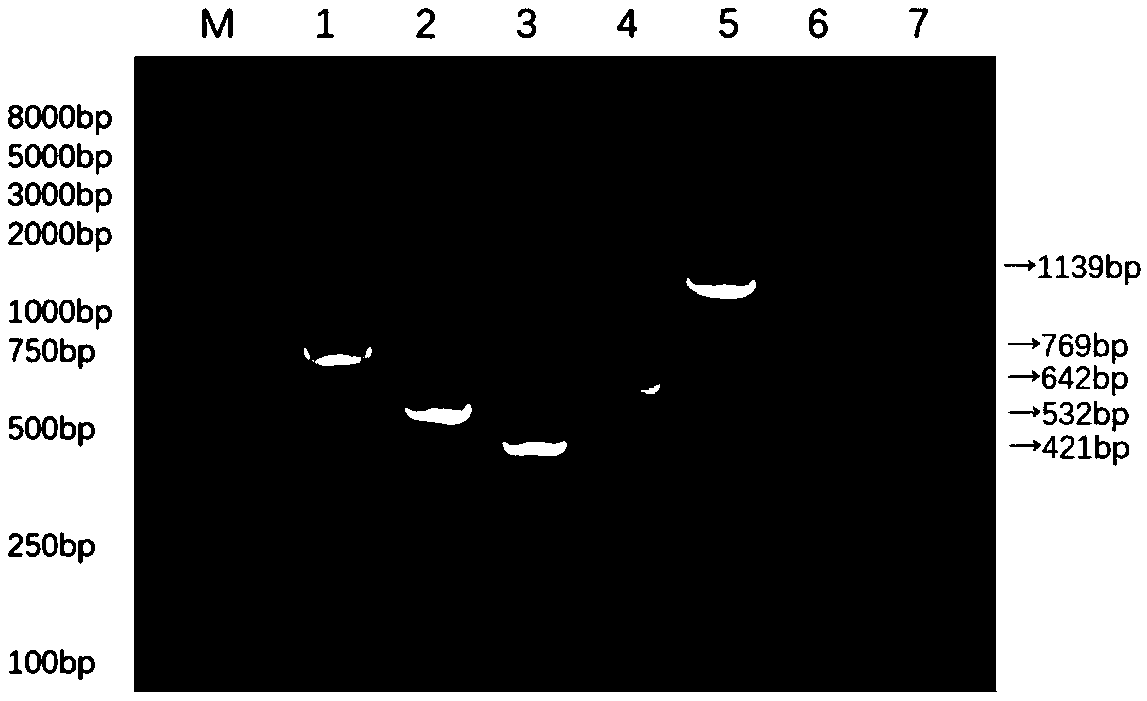
[0049] Example 1 Primer Design
[0050] The specific primer pairs designed to detect enterotoxigenic Escherichia coli F4, F5, F6, F41, and F18 pilus genes are as follows:
[0051] The primer pair used to detect the F4 pili gene of enterotoxigenic Escherichia coli is:
[0052] F1: 5'-ATTTCAATGGTTCGGTCG-3' (shown in SEQ ID NO.1)
[0053] R1: 5'-GATTGCTACGTTCAGCGGAGCG-3' (shown in SEQ ID NO.2)
[0054] The primer pair used to detect the F5 pili gene of enterotoxigenic Escherichia coli is:
[0055] F2: 5'-AAACACTGCTAGCTATTATC-3' (shown in SEQ ID NO.3)
[0056] R2: 5'-ATAAGTGACTAAGAAGGATGC-3' (shown in SEQ ID NO.4)
[0057] The primer pair used to detect the F6 pili gene of enterotoxigenic Escherichia coli is:
[0058] F3: 5'-ACTTCTAATCTGTCGCAAACC-3' (shown in SEQ ID NO.5)
[0059] R3: 5'-GAACGAATAGTCATTACTGCAC-3' (shown in SEQ ID NO.6)
[0060] The primer pair used to detect the pili gene of enterotoxigenic Escherichia coli F41 is:
[0061] F4: 5'-ATCAGCGGCAGTATCTGGTT-3' (...
Embodiment 2
[0067] The preparation of embodiment 2 kit
[0068] Described test kit comprises each component shown in following table 1:
[0069] Table 1
[0070]
[0071]
[0072] Wherein, the positive quality control standard product is prepared according to the following method: PCR method is used to amplify enterotoxigenic E. coli F4, F5, F6, F41, F18 pilus genes or partial genes respectively, and the products are respectively combined with the cloning vector pMD- 19-T connection, transforming competent Escherichia coli Trans T1 to obtain positive recombinant bacteria, and preparing positive plasmids according to the instructions of Zhongkeruitai Ordinary Plasmid Mini-prep Kit.
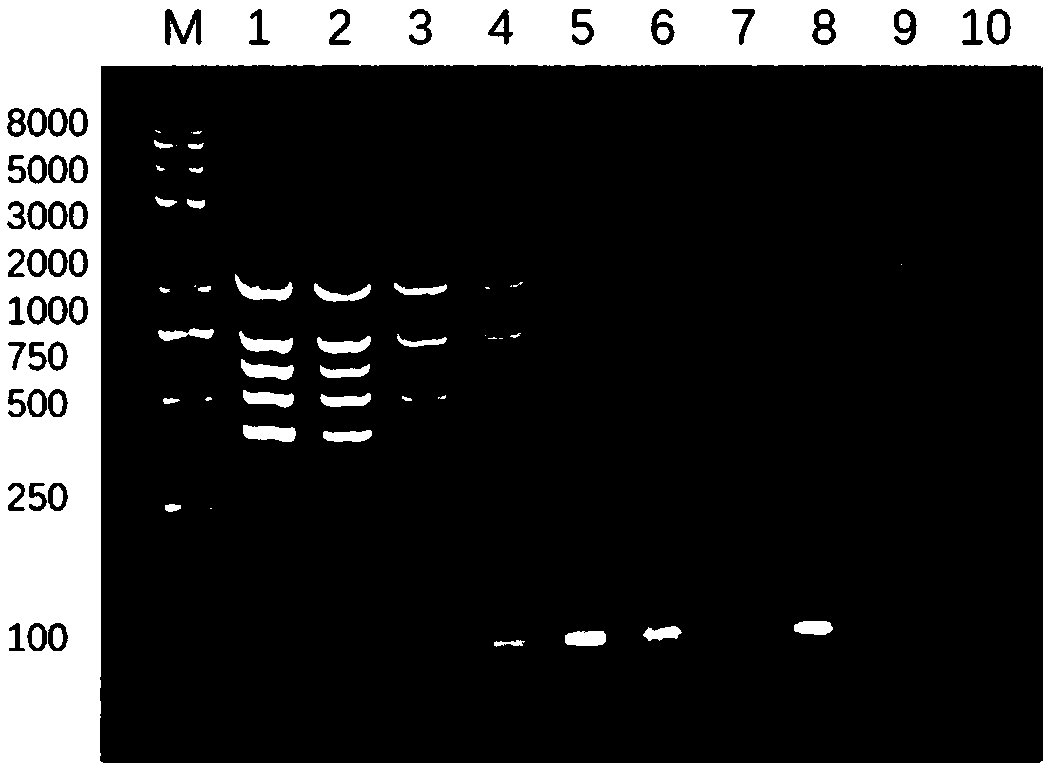
Embodiment 3
[0073] Example 3 Establishment of Multiplex PCR Detection Method for Enterotoxigenic Escherichia coli F4, F5, F6, F41, F18 Pili Genes
[0074] 1. Establishment of Multiplex PCR Detection Method
[0075] (1) Preparation of Escherichia coli DNA template
[0076] Coliform reference strain C83903 (F4 + ), C83199 (F5 + ), C83915 (F6 + ), C83920 (F5 + 、F41 + ), C83684 (F18 + ) inoculated into LB liquid medium, and enriched overnight at 37°C. Take 1 mL of each reference strain culture in a 1.5 mL centrifuge tube, centrifuge at 10,000×g for 5 min, remove the supernatant, add 100 μL of sterilized deionized water to resuspend, boil at 100°C for 10 min, and immediately place in ice-water mixture Cool for 5 minutes, then centrifuge at 10,000×g for 5 minutes, then take the supernatant and store it at -20°C, and use it as a DNA template for later use.
[0077] (2) Multiplex PCR amplification reaction system and composition
[0078] Prepare a multiplex PCR reaction system with a tot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com