Preparation method of oxaziclomefone
A technology of oxaziclozone and its compound, which is applied in the field of preparation of oxaziclozone, can solve the problems of low yield and cumbersome synthesis process, and achieve the effects of high yield, shortened synthetic route, and good stability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
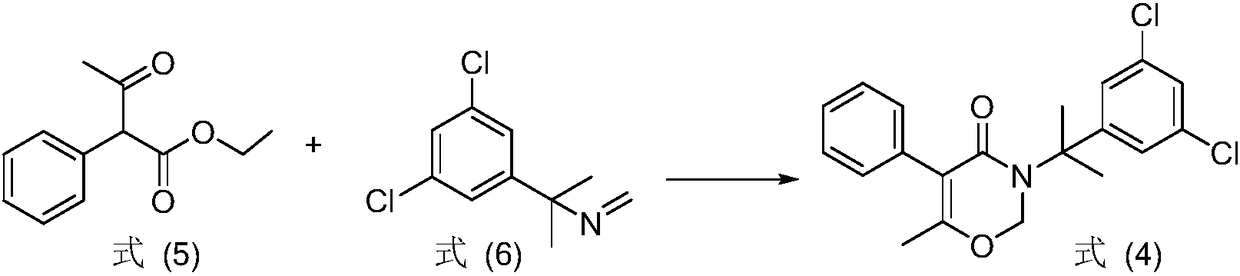
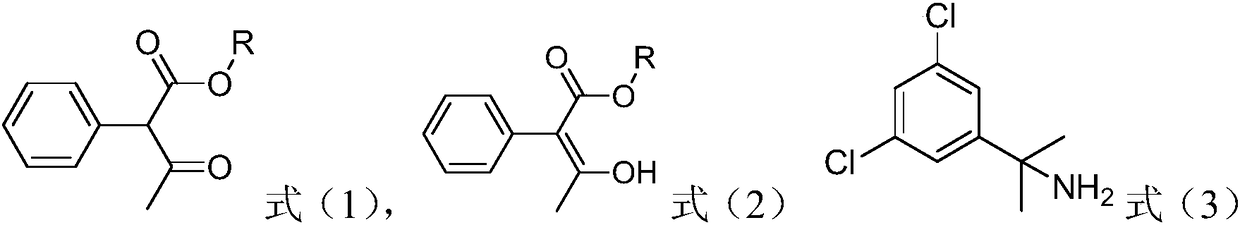
[0017] The invention provides a method for preparing oxaziclozone, the method comprising the following steps: in the presence of paraformaldehyde and a catalyst, combining the compound represented by formula (1) or formula (2) with the compound represented by formula (3) Shown 1-methyl-1-(3,5-dichlorophenyl) ethylamine reacts;
[0018]
[0019] Among them, R is C 1 -C 5 the alkyl group;
[0020] The general formula of the paraformaldehyde is—(CH 2 O) n —, n is an integer of 2-100;
[0021] The catalyst contains alcohol, alkali and free radical inhibitor.
[0022] In the present invention, the compounds represented by the formula (1) and formula (2) are isomers of each other, and both are keto and enol isomers. The C 1 -C 5 The alkyl group includes, but is not limited to: at least one of methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, n-pentyl, isopentyl and tert-amyl . Preferably, R is methyl or ethyl.
[0023] In the present invention, the par...
Embodiment 1
[0039] This example is used to illustrate the preparation method of oxaziclozone of the present invention
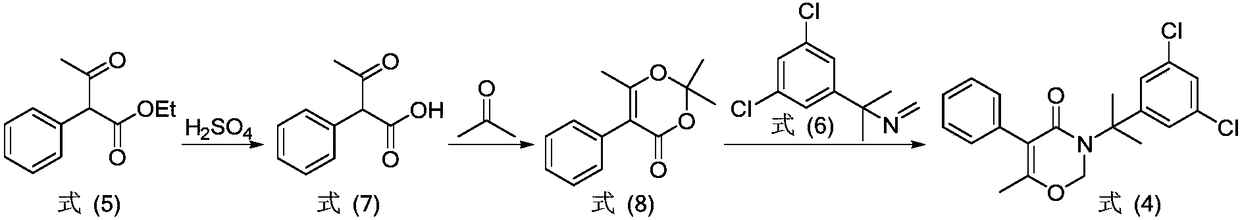
[0040] Paraformaldehyde (0.88g), ethanol (1.35g), triethylamine (0.02g), Na 2 S (0.015 g), 1-methyl-1-(3,5-dichlorophenyl)ethylamine (2 g) were added to a 200 mL reaction flask, and 100 mL of xylene was added thereto. After the reaction solution was heated to reflux for 5 h under stirring, 1-acetophenethyl ester (2.1 g) was added thereto and refluxed at 180° C. for 6 h. After the reaction was completed, the system was cooled to room temperature, xylene was removed under reduced pressure, and the obtained residue was recrystallized from petroleum ether (15 mL). The solid obtained by recrystallization was suction-filtered and vacuum-dried to obtain 3 g of oxaziclozone as a white solid with a yield of 81%.
[0041] 1 H NMR (400MHz, CDCl 3 )δ7.27 (m, 8H), 5.45 (s, 2H), 1.89 (s, 3H), 1.64 (s, 6H).
Embodiment 2
[0043] This example is used to illustrate the preparation method of oxaziclozone of the present invention
[0044] Paraformaldehyde (1.47g), methanol (0.5g), N-methylpyrrole (0.4g), hydroquinone (0.015g), 1-methyl-1-(3,5-dichlorophenyl ) Ethylamine (2g) was added into a 200mL reaction flask and 100mL of toluene was added thereto. After the reaction solution was heated to reflux for 5 h under stirring, 1-acetylbenzyl ester (4.7 g) was added thereto and refluxed at 110° C. for 10 h. After the reaction was completed, the system was cooled to room temperature, and the toluene was removed under reduced pressure, and the obtained residue was recrystallized from petroleum ether (15 mL). The solid obtained from the recrystallization was suction filtered and vacuum dried to obtain 2.95 g of oxaziclozone as a white solid with a yield of 80%.
[0045] 1 H NMR (400MHz, CDCl 3 )δ7.27 (m, 8H), 5.45 (s, 2H), 1.89 (s, 3H), 1.64 (s, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com