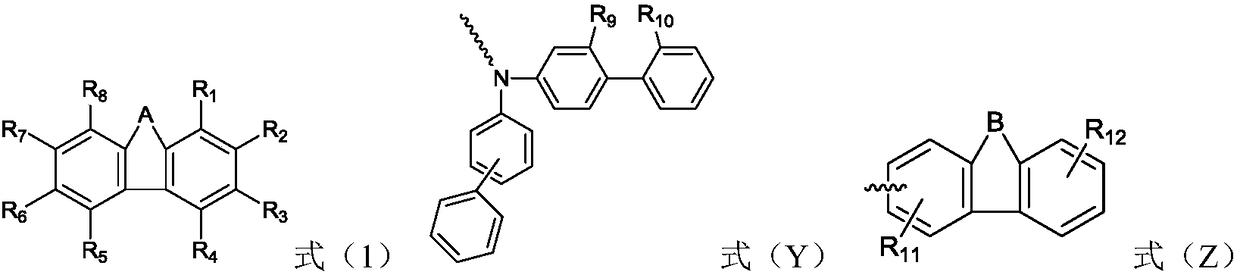
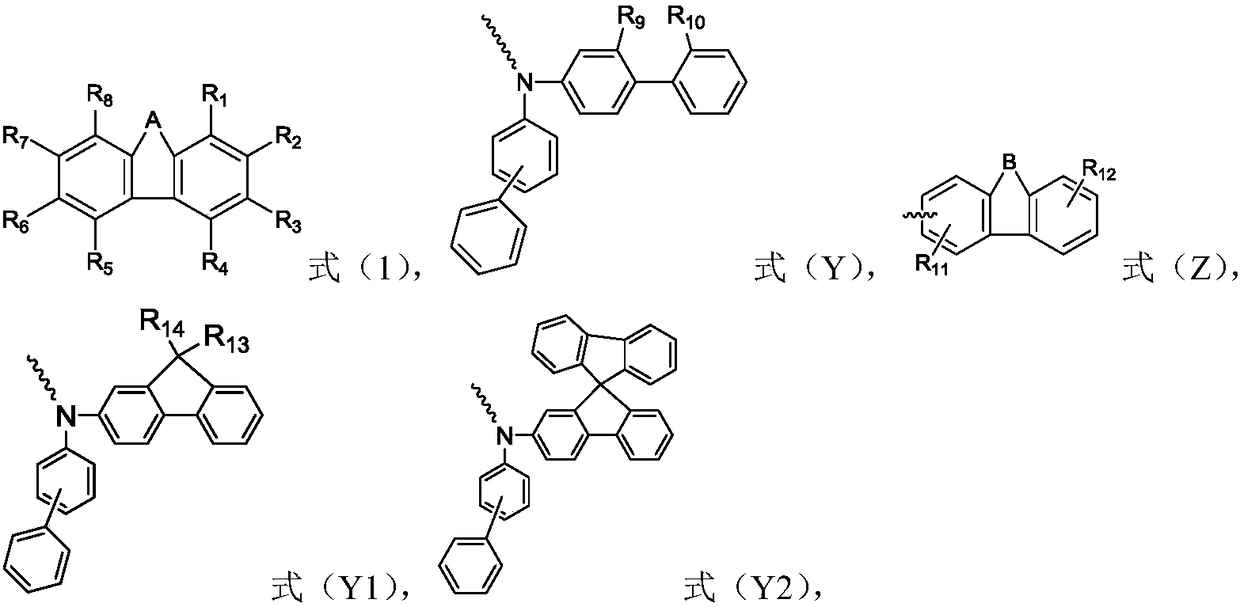
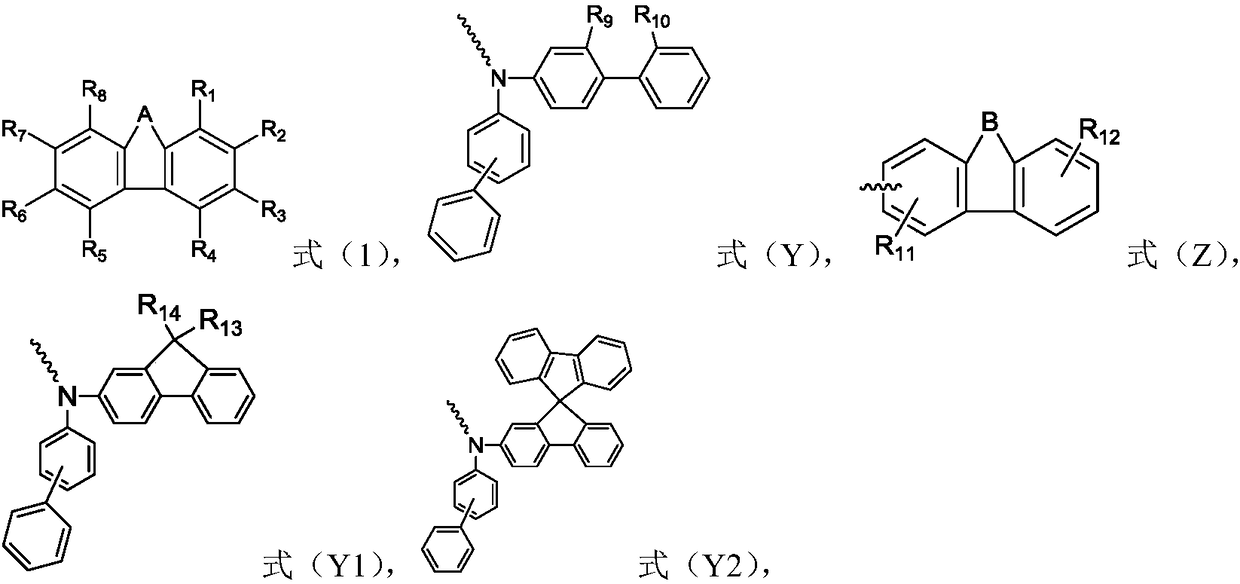
Organic compound and organic electroluminescence device with same
An electroluminescent device and organic compound technology, applied in the field of organic electroluminescent devices, can solve the problems of low driving voltage, low current efficiency and brightness, and short service life, and achieve low driving voltage, high current efficiency and brightness, The effect of long service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0058] Synthesis Example 1: Synthesis of Compound 1
[0059]
[0060] Synthesis of Intermediate 1-1: Dissolve 0.1926mol of 2-bromo-4-iododibenzofuran (TCI-Tieri (Shanghai) Chemical Industry Development Co., Ltd.) in 720mL of 1,4-dioxane In the solvent, stir under nitrogen gas, add 0.1926mol of dibenzofuran-4-boronic acid, 0.3852mol of potassium carbonate, 53mL of water, 0.0019mol of tetrakis(triphenylphosphine) palladium in sequence, heat up to reflux reaction, and detect the raw materials after 3h After the reaction was completed, the reaction solution was spin-dried under reduced pressure, and 51.74 g of intermediate 1-1 was obtained by column chromatography (the yield was 65% by weight). Calculated value C 24 h 13 BrO 2 : 413.26+1.
[0061] The nuclear magnetic data of intermediate 1-1 is: 1H-NMR (400MHz, CDCl 3 ) (ppm) δ = 7.36 ~ 7.42 (5H, m), 7.69 ~ 7.72 (2H, d), 7.82 ~ 7.94 (4H, m), 8.12 ~ 8.15 (2H, d).
[0062] Synthesis of compound 1: Dissolve 0.1024 mol of i...
preparation example 2
[0064] Preparation Example 2: Synthesis of Compound 3
[0065]
[0066] Intermediate 3-1 and compound 3 were synthesized by a method similar to that of Preparation Example 1 (only the types of raw materials used were different).
[0067] 16.5 g of Intermediate 3-1 was obtained (yield 63% by weight). Calculated value C 27 h 19 BrO: 439.34+1.
[0068] The nuclear magnetic data of intermediate 3-1 is: 1H-NMR (400MHz, CDCl3) (ppm) δ = 1.72 ~ 1.75 (6H, s), 7.36 ~ 7.42 (5H, m), 7.52 ~ 7.53 (2H, m), 7.69~7.72 (1H, m), 7.82~7.94 (3H, m), 8.15~8.18 (2H, d).
[0069] And 9.6 g of compound 3 was obtained (yield 53% by weight). Calculated value C 51 h 37 NO: 679.85+1.
[0070] The nuclear magnetic data of compound 3 is: 1H-NMR (400MHz, CDCl 3 ) (ppm) δ = 1.72-1.75 (6H, s), 6.72-6.75 (4H, d), 7.35-7.77 (24H, m), 7.92-8.05 (3H, m).
preparation example 3
[0071] Preparation Example 3: Synthesis of Compound 46
[0072]
[0073] Intermediate 46-1 and compound 46 were synthesized by a method similar to that of Preparation Example 1 (only the types of raw materials used were different).
[0074] 23.1 g of intermediate 46-1 were obtained (yield 61% by weight). Calculated value C 27 h 19 BrO: 439.34+1.
[0075] The NMR data of intermediate 46-1 are: 1H-NMR (400MHz, CDCl3) (ppm) δ = 1.72 ~ 1.75 (6H, s), 7.32 ~ 7.56 (4H, m), 7.56 ~ 7.59 (1H, d), 7.69-7.75 (3H, m), 7.79-7.82 (1H, s), 7.87-7.93 (3H, m), 8.12-8.15 (1H, s).
[0076] And 15 g of compound 46 was obtained (yield 49% by weight). Calculated value C 51 h 37 NO: 679.85+1.
[0077] The NMR data of compound 46 are: 1H-NMR (400MHz, CDCl3) (ppm) δ = 1.72 ~ 1.75 (6H, s), 6.72 ~ 6.75 (4H, d), 7.07 ~ 7.10 (1H, s), 7.32 ~ 7.64 (20H, m), 7.77-7.80 (1H, s), 7.87-7.93 (3H, m).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com