Application of E-2-styryl benzimidazole compound to preparation of drugs resisting hepatitis B virus
A benzimidazole and styryl technology, applied in the application field of ethanol compounds, can solve the problems of poor tolerance, high cost, many side effects, etc., and achieve the effects of good antiviral effect, wide application prospect, and no toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
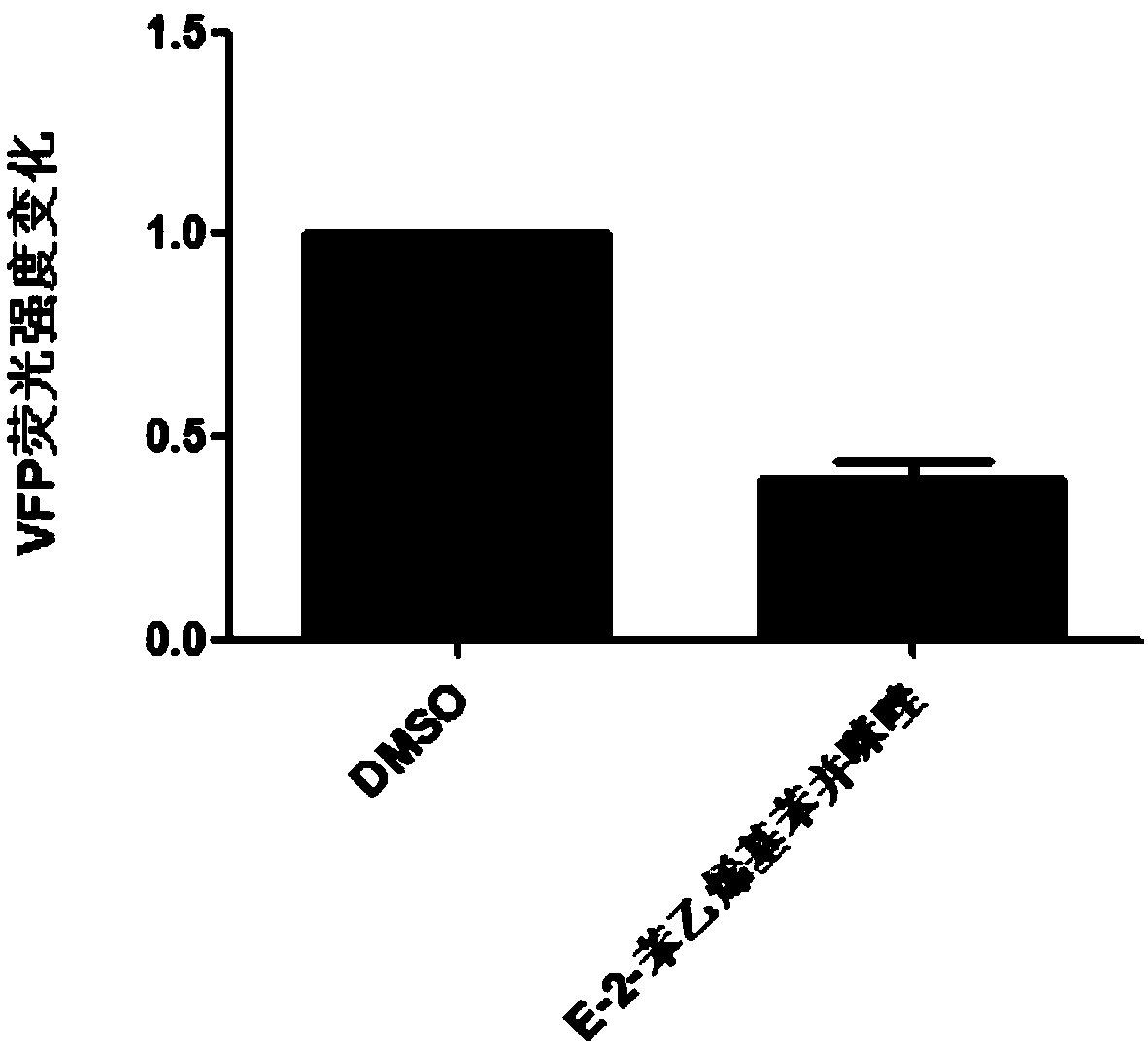
[0025] Study on the Inhibitory Effect of E-2-Styrylbenzimidazole on Interbinding of HBV c Antigen
[0026] Experimental method: Take well-grown human adrenal cell line 239t cells and inoculate them in a 96-well transparent flat-bottomed plate, with 5×104 cells per well. The medium used is complete medium: high-glucose DMEM, 10% fetal bovine serum and 1% double antibody, the culture condition is 5% carbon dioxide, 37°C; C and pcDNA3.1-HBVcAg-VFP-N two plasmids. For transfection, liposome-encapsulated transfection was used, lipo2000 was used as the reagent, and 20 μl of transfection solution was used. After 4 hours of transfection, the compound to be screened was added, 2 μl per well, with a final concentration of 50 μM. After culturing for 48 hours, the expression of green fluorescent protein VFP was detected. If there is a decrease in the expression of green fluorescent protein VFP, the compound may become an antiviral drug candidate. Experimental results such as figure 1...
Embodiment 2
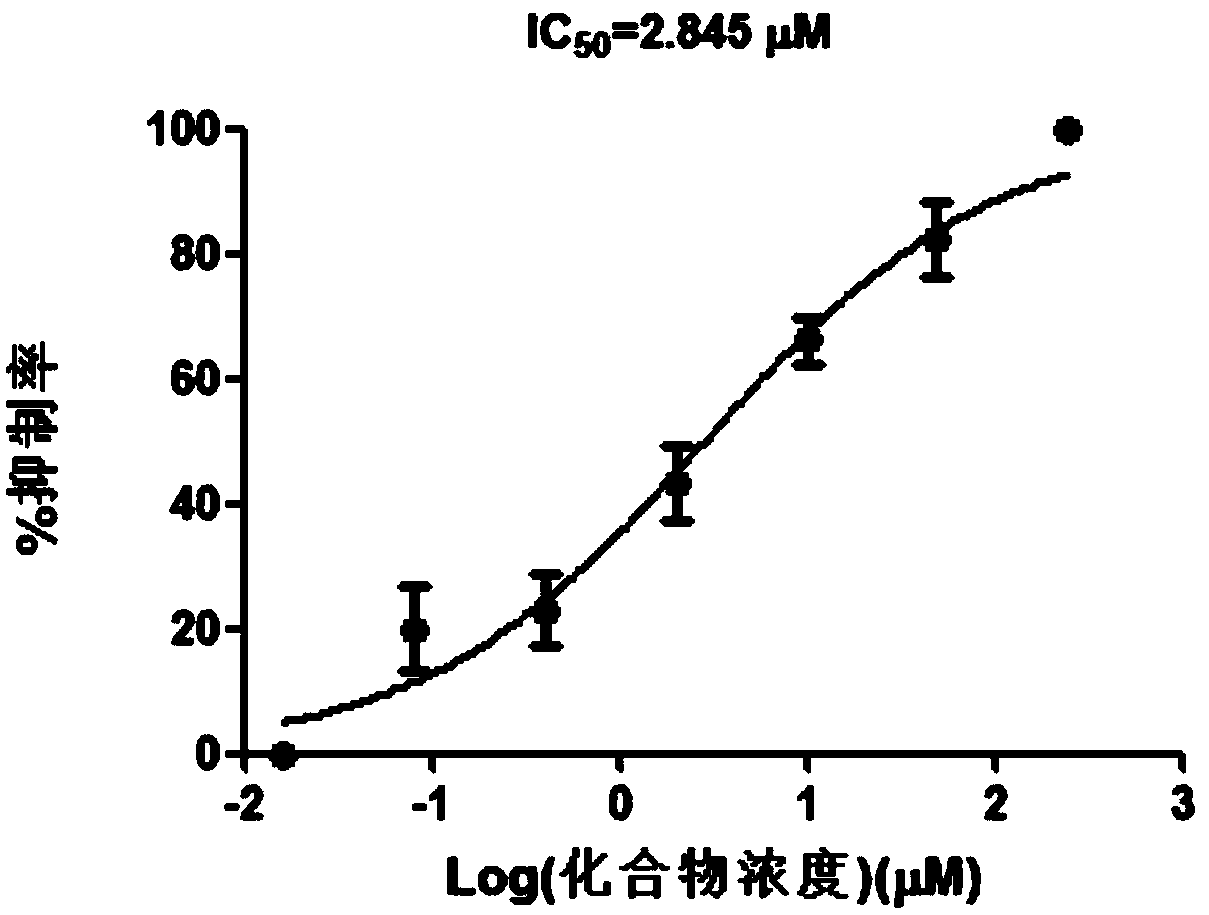
[0028] E-2-Styrylbenzimidazole inhibits viral replication of wild-type HBV
[0029] Experimental method: E-2-styrylbenzimidazole compounds of the present invention wild-type HBV virus replication research: take the cell line HepG2.2.15 that grows well and can produce wild-type HBV virus, the amount of cells used is 2×104 / well, 24 hours after the 96-well plate was plated, different concentrations of compounds were added, 2 μl of the compound per well (final concentration 250 μM, 50 μM, 10 μM, 2 μM, 0.4 μM, 0.08 μM, 0.016 μM, 0 μM); use 2% Triton X-100 to process and collect The supernatant of the cells was collected and cultured for 8 days, and then the DNA content of HBV in the cell culture supernatant was detected. Experimental results such as figure 2 shown. It can be seen from the experimental results that E-2-styrylbenzimidazole has a good effect on inhibiting the replication of wild-type HBV virus, and its IC50 is 2.845 μM.
Embodiment 3
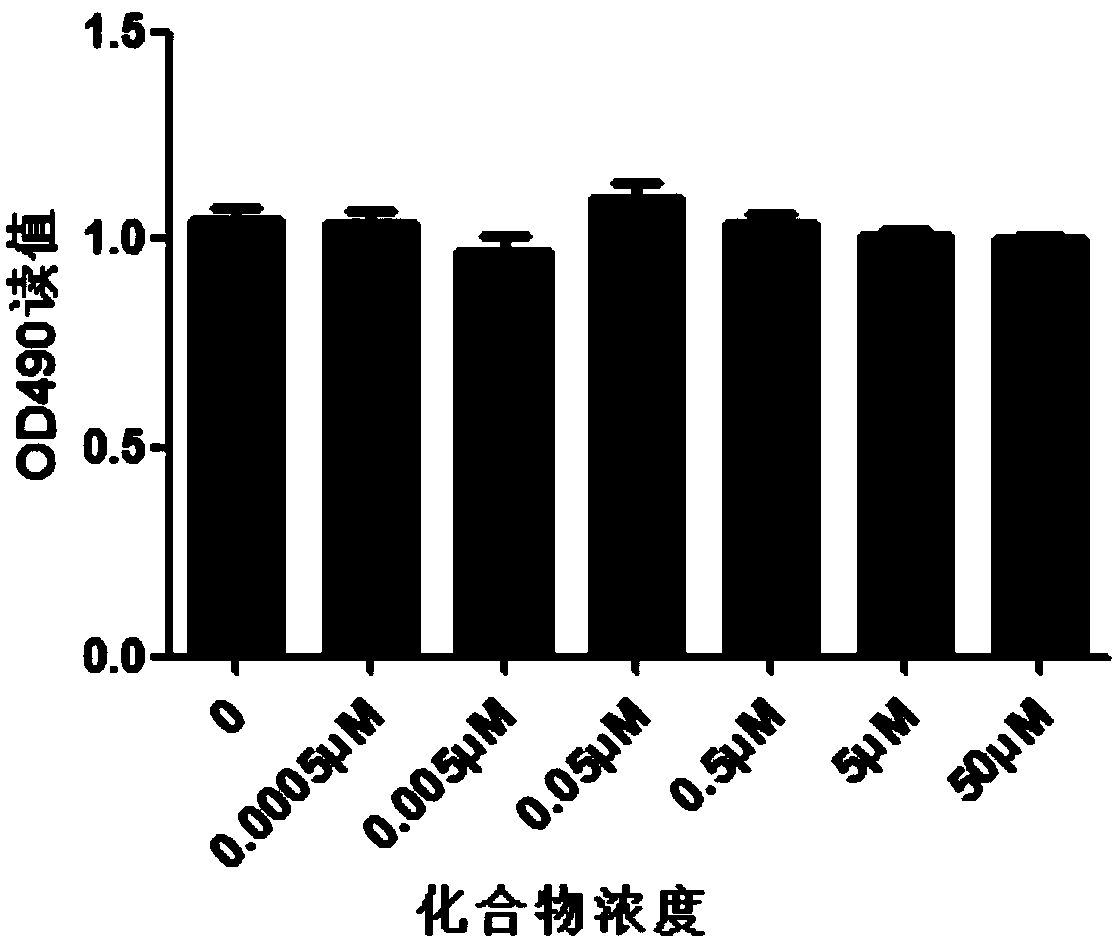
[0031] Cytotoxicity of E-2-Styrylbenzimidazole in 293t Cells
[0032] Experimental method: inoculate cells, use DMEM medium containing 10% fetal calf serum to prepare 293t into a single cell suspension, inoculate 1000 cells per well into a 96-well plate with a volume of 200ul per well; add the compound after 24 hours of adherence , 2 μl per well, the final concentrations were 50 μM, 5 μM, 0.5 μM, 0.05 μM, 0.005 μM, 0.0005 μM, 0 μM; after 48 hours of culture, add 20 μl of MTS solution to each well, and continue to incubate in the incubator for 2 to 4 hours; choose 490 nm wavelength, the light absorbance value of each well was measured on an enzyme-linked immunosorbent monitor, and the cytotoxicity of the compound to 293t cells was observed. Experimental results such as image 3 shown. From the experimental results, it can be seen that E-2-styrylbenzimidazole has low toxicity and shows no cytotoxicity in 293t cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com