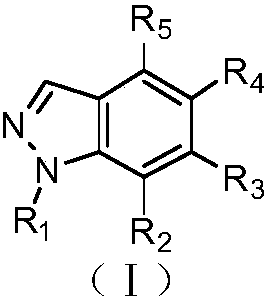
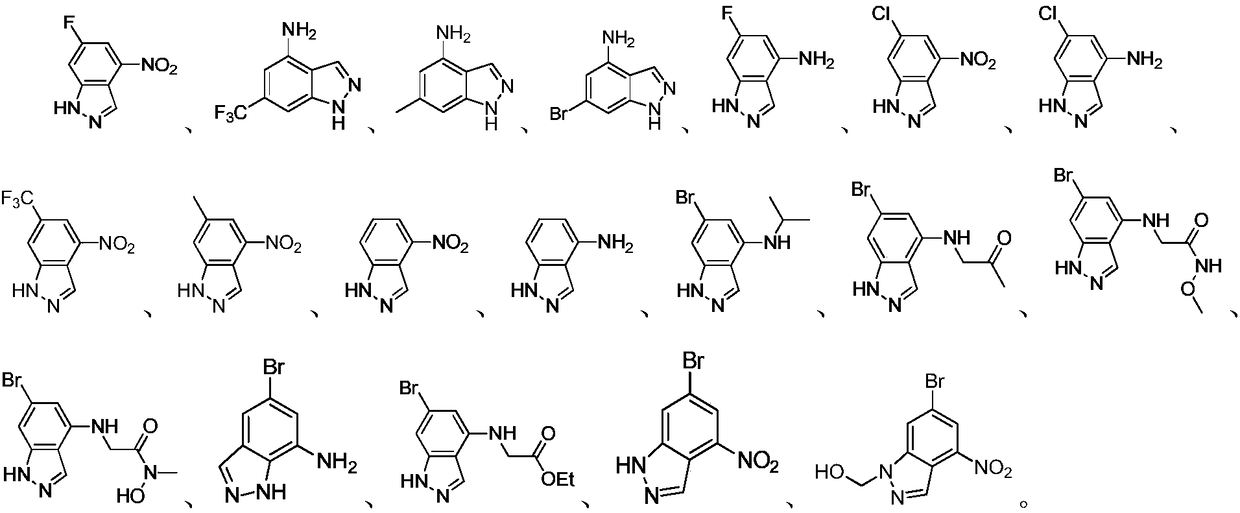
Indazole compounds containing nitrogen substituents, and application of same as IDO inhibitors
A compound and solvate technology, applied in the field of IDO inhibitors, can solve the problems of reducing tryptophan concentration, stagnation of synthesis, inhibiting killing effect, etc., and achieving excellent inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
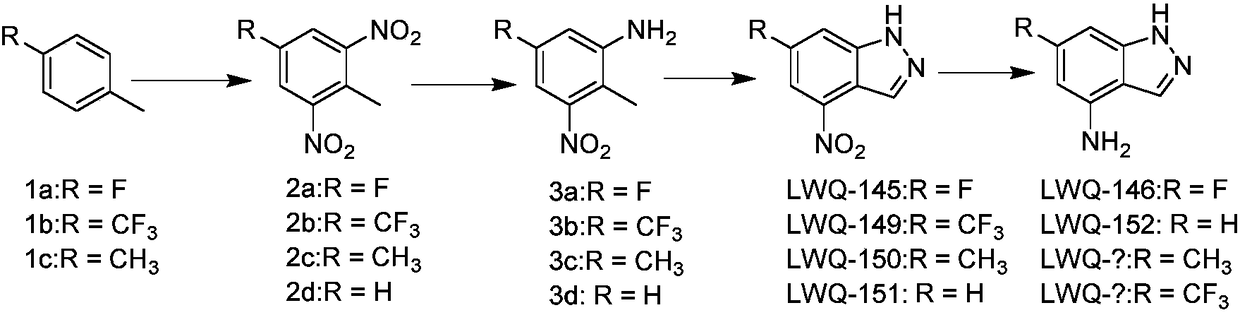
[0045] The synthesis of embodiment 1 compound LWQ-121, LWQ-122, LQW-145, LQW-146, LQW-149, LQW-150, LQW-151 and LQW-152
[0046] The synthetic route is as follows:
[0047]
[0048] (1) Synthesis of Compound LWQ-145
[0049] 1 Synthesis of compound 2a:
[0050] In an ice bath, the KNO 3(1.146g, 11.34mmol) was slowly added to compound 1a (500mg, 4.53mmol). After the addition was complete, it was moved to room temperature and stirred for 12h. TLC showed that the reaction of the raw materials was complete, and finally 50mg of a white solid was obtained. Yield: 5.5%.
[0051] 1 H NMR (400MHz, CDCl 3 ,ppm): δ8.36(d,J=7.9Hz,2H,),2.41(s,3H).
[0052] 2 Synthesis of Compound 3a:
[0053] The raw material 2a (200mg, 1mmol) was dissolved in methanol (5mL) 1,4-dioxane (2.5mL), and 10N HCl (1mL) Fe (41.9mg, 3mmol) was added under ice-cooling. After the addition was complete, it was stirred at 80°C for 3h. TLC showed the starting material was completely reacted. Finally, 42 mg o...
Embodiment 2
[0070] The synthesis of embodiment 2LQW-123, LQW-147, LQW-148, LWQ-221 and LWQ-222
[0071] The synthetic route is as follows:
[0072]
[0073] (1) Synthesis of Compound LWQ-145
[0074] 1 Synthesis of compound 4a:
[0075] At room temperature, the raw material TCCA (640mg, 2.74mmol) was slowly added to the concentrated sulfuric acid (25mL) solution of 2d (1g, 5.49mmol). Powder 872.6 mg, yield: 73.6%.
[0076] 2 Synthesis of Compound 5a:
[0077] Dissolve raw material 4a (5.9g, 23.60mmol) in methanol (10mL) 1,4-dioxane (5mL), add 10N HCl (12mL) Fe (3.96g, 70.80mmol) under ice-cooling, after addition, 80°C After stirring for 3h, TLC showed that the raw material was completely reacted. Finally, 3.55 g of light yellow powder was received, yield: 68.4%.
[0078] 3 Synthesis of compound LWQ-147:
[0079]The raw material 5a (3.55g, 16.13mmol) was dissolved in AcOH (15mL), and an aqueous solution (5mL) of sodium nitrite (2.23g, 32.26mmol) was added at 0°C. After the additi...
Embodiment 3
[0092] The synthesis of embodiment 3 compound LQW-161
[0093] The synthetic route is as follows:
[0094]
[0095] 1 Synthesis of Intermediate 8
[0096] Dissolve compound 7 (50 mg, 0.24 mmol) and compound acetone (85 μL, 1.18 mmol) in 1.5 mL of dichloroethane, add molecular sieves, and place in a microwave reactor. Set the power to 650W and the time to 540min. After the reactor was stopped, the molecular sieves were filtered out, and the molecular sieves were washed with dichloromethane for several times. The filtrates were combined and concentrated, and the crude product was purified by column chromatography to obtain compound 8 (38 mg, 0.16 mmol) as a white solid with a yield of 67%.
[0097] 2 Synthesis of compound LWQ-161
[0098] Compound 8 (30 mg, 0.16 mmol) and DMF (3 μL, 0.03 mmol) were dissolved in dichloromethane and placed at 0°C. Under the protection of Ar gas, add diluted HSiCl 3 (20 μL, 0.19 mmol) in dichloromethane. After addition, stir at the same t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com