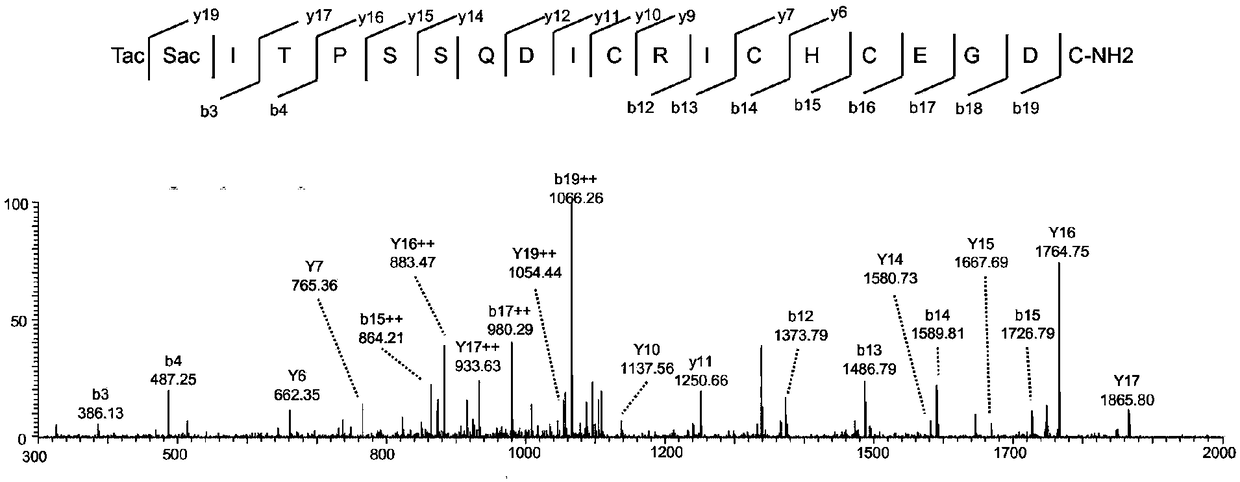
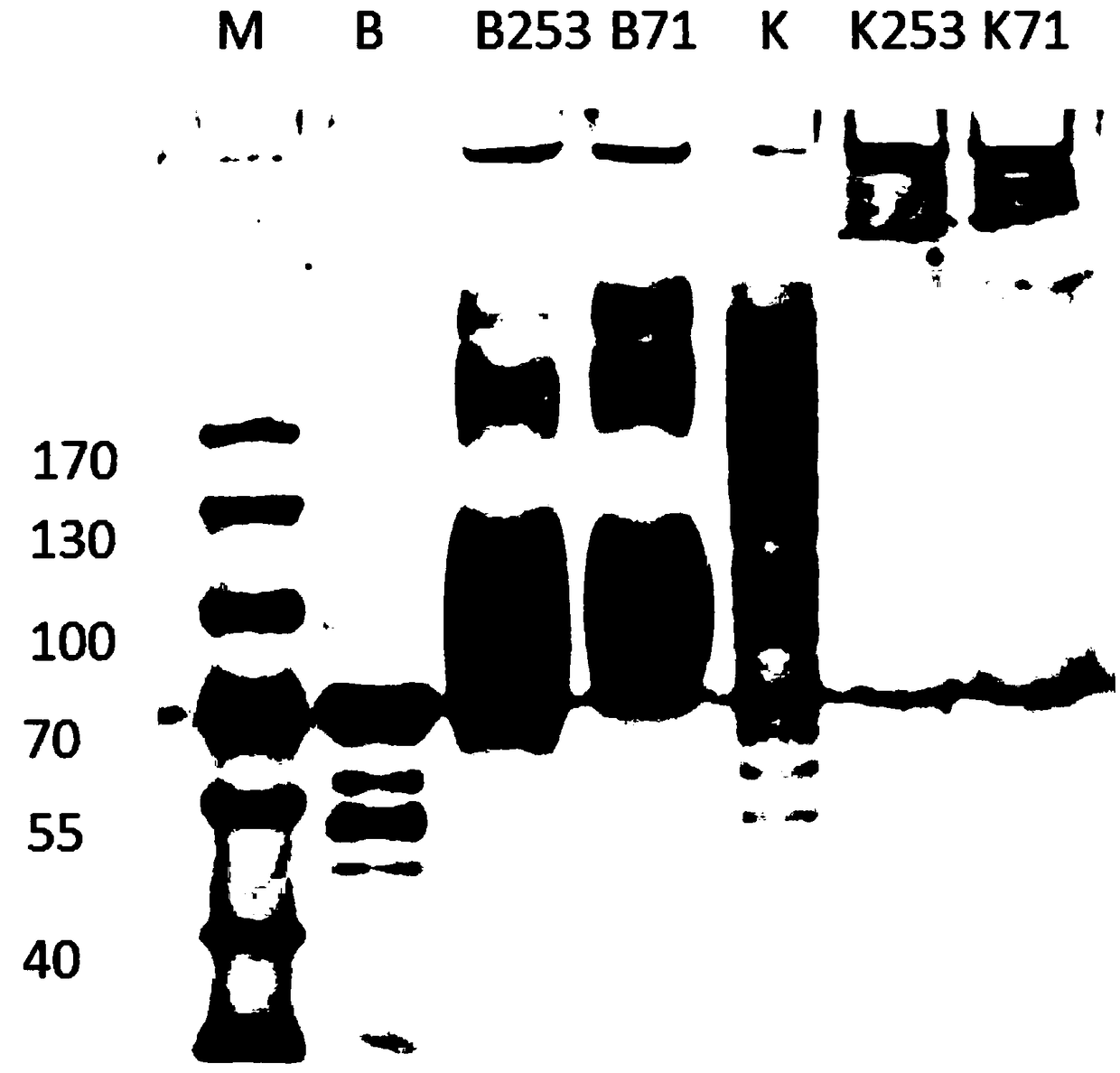
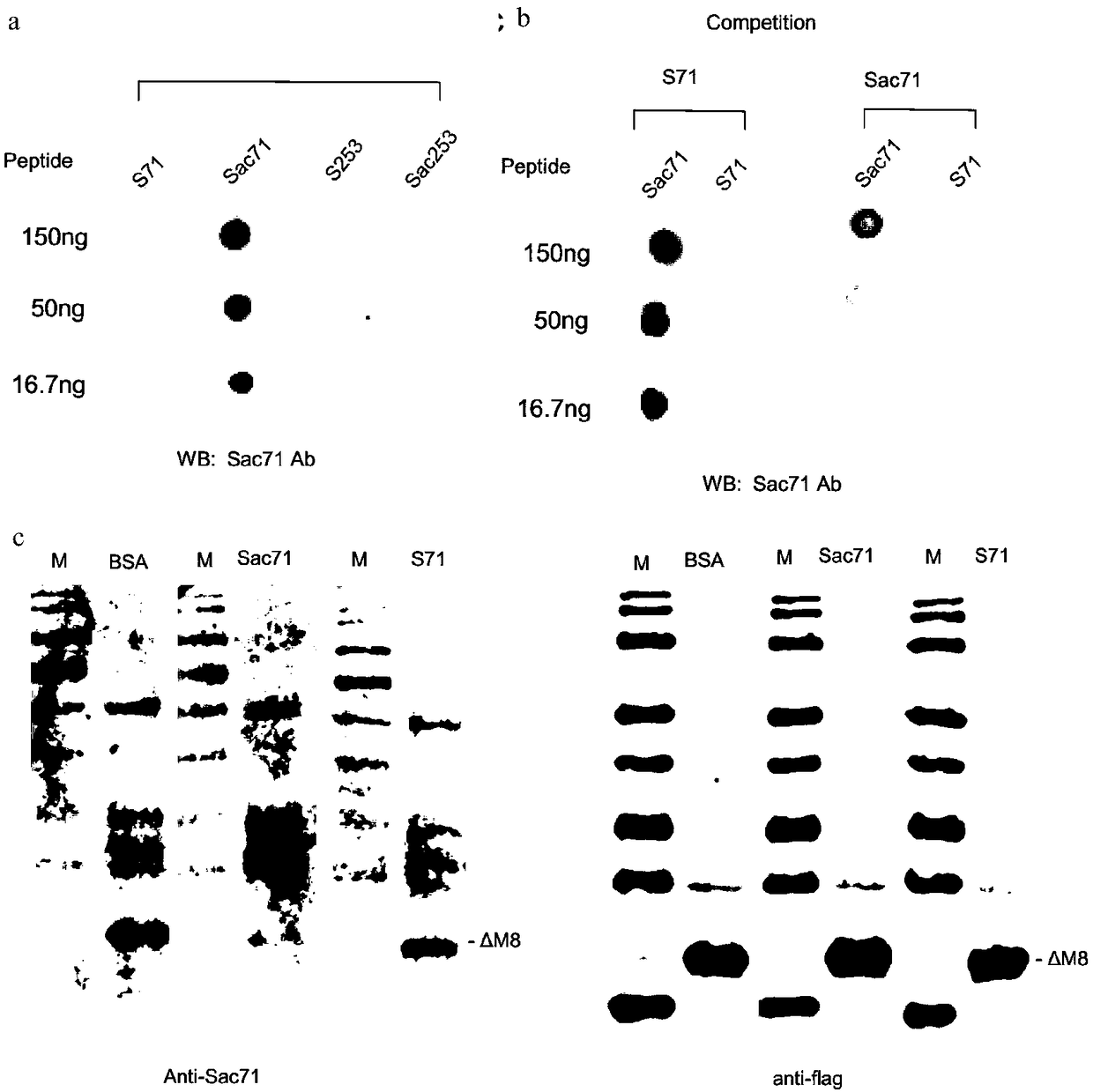
M8Sac71 antibody as well as preparation method and application thereof
An m8sac71, antibody technology, applied in the direction of anti-enzyme immunoglobulin, serum immunoglobulin, measuring device, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1M8
[0039] Preparation and detection of embodiment 1M8Sac71 antibody
[0040] 1. Materials and instruments:
[0041] (1) Purification buffer:
[0042] Disodium hydrogen phosphate (Na 2 HPO 4 .12H 2 O), 29.01g;
[0043] Sodium dihydrogen phosphate (NaH 2 PO 4 .2H 2 O), 2.964g;
[0044] Sodium chloride, 9.00g;
[0045] wxya 2 0 to 1 L, pH = 7.4.
[0046] (2) Conjugation buffer: Purification bu+5mM EDTA.
[0047] (3) 5 mg BSA, Sigma A1933.
[0048] (4) Sulfo-SMCC (5 mg / ml) Thermo Scientific #22322, 50 mg.
[0049] (5) Freund's complete adjuvant and Freund's incomplete adjuvant: Sigma products.
[0050] (6) A glass syringe (10ml) with a good seal.
[0051] (7) Surgical instruments: several sets of scalpels, scissors, and tweezers.
[0052] (8) Artery clips, blood collection tubes, syringes, etc.
[0053] (9) Anesthetic: Chloral hydrate (10%).
[0054] (10) ELISA related reagents: 0.1M Na 2 CO 3 (PH=9.6), TBST, etc.
[0055] (11) Goat anti-rabbit antibody labeled w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com