Polycarbonyl nitrogen heterocyclic organic compound for organic cathode material of lithium battery and preparation method thereof
A technology of organic compounds and heterocyclic compounds, which is applied in the field of preparation of lithium battery electrode materials, can solve problems such as poor electrical conductivity and easy dissolution loss, and achieve the effects of excellent performance, good reproducibility, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
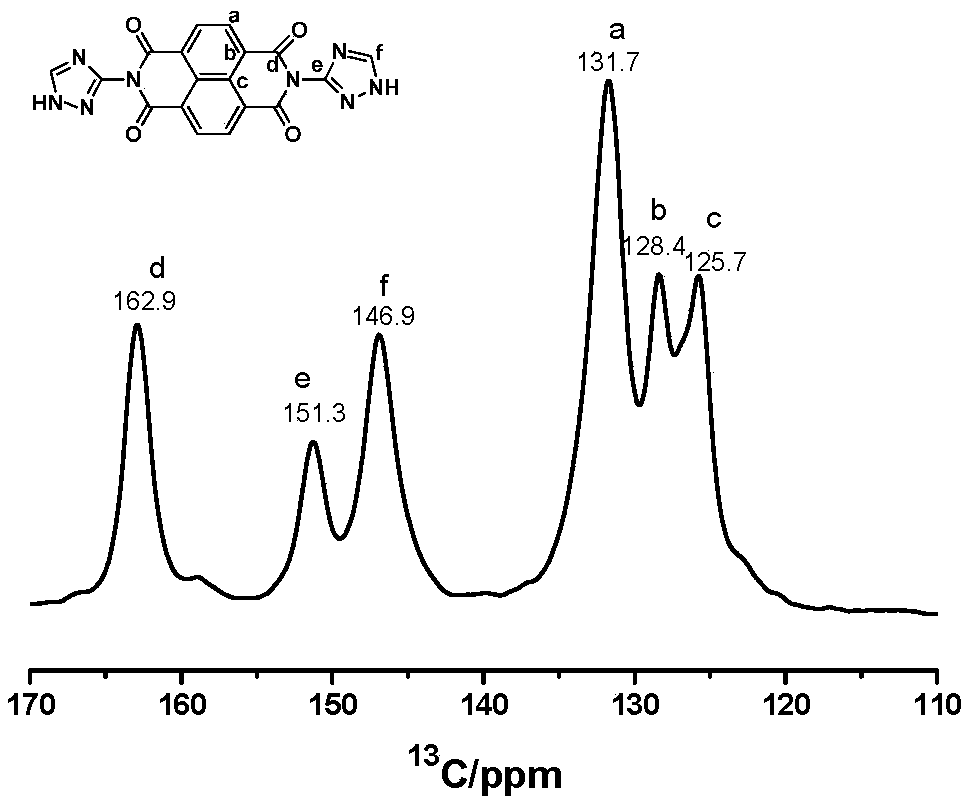
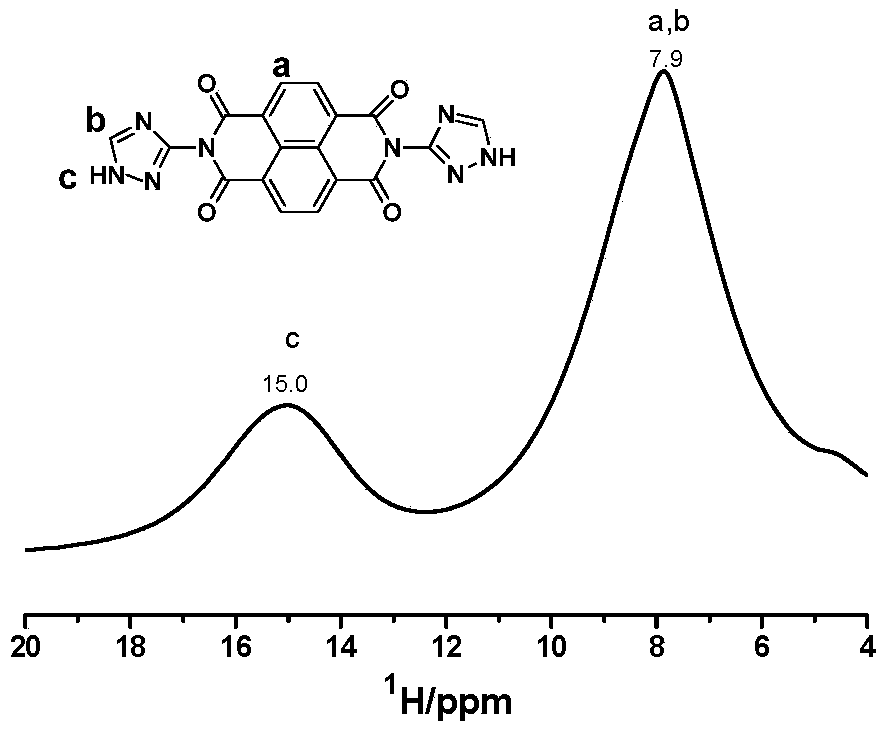
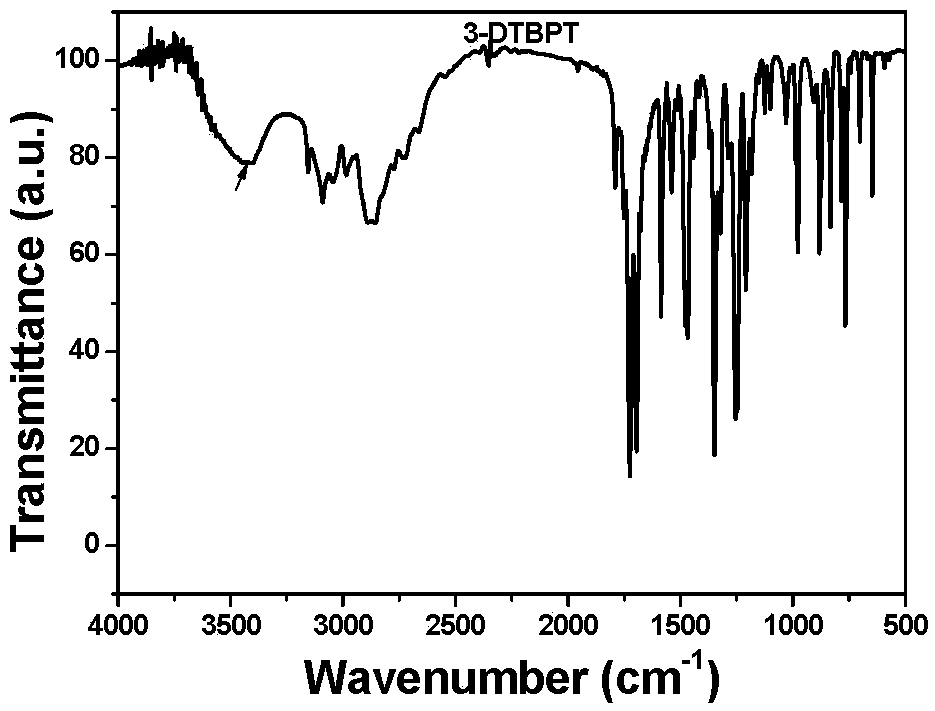
[0034] First, 70 mL of N,N'-dimethylformamide (DMF) was added to a round-bottomed flask equipped with a condenser and a stirring device. Weighed 1,4,5,8-naphthalenetetracarboxylic anhydride (3mmol, 0.805g) and 3-amino-1,2,4-triazole (6mmol, 0.504g) were added to the previous DMF solution under nitrogen protection In the process, a suspension is obtained by stirring, and then the suspension is heated to 150°C in an oil bath under the protection of nitrogen or argon, and the reaction is carried out for 15h. The whole process requires continuous stirring and reflux. Finally, the oil bath was removed, and after the temperature of the solution dropped to room temperature, the product was precipitated through an ice bath, washed with water, and filtered to collect the gray-green solid product Compound A. 1 H NMR spectrum ( figure 1 ), 13 C nuclear magnetic spectrum ( figure 2 ),Infrared spectra( image 3 ) indicates that the synthesized product is A. Heat Gravity Map ( Figur...
Embodiment 2
[0036] The experimental method is the same as in Example 1, except that the 3-amino-1,2,4-triazole is changed to 4-amino-4H-1-2-4-triazole (6mmol, 0.504g) to obtain compound C .
Embodiment 3
[0038] The experimental method was the same as that of Example 1, except that 3-amino-1,2,4-triazole was changed to 2-aminoimidazole (6 mmol, 0.498 g) to obtain compound E.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com