Titanium dioxide nanoflower rich in oxygen vacancies and preparation method of titanium dioxide nanoflower
A technology of titanium dioxide and nanoflowers, applied in chemical instruments and methods, catalyst activation/preparation, inorganic chemistry, etc., can solve problems such as poor monodispersity of titanium dioxide nanoflowers, weak light absorption of titanium dioxide, wide product size distribution, etc., to broaden the absorption range, increased multiple scattering performance, and the effect of simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
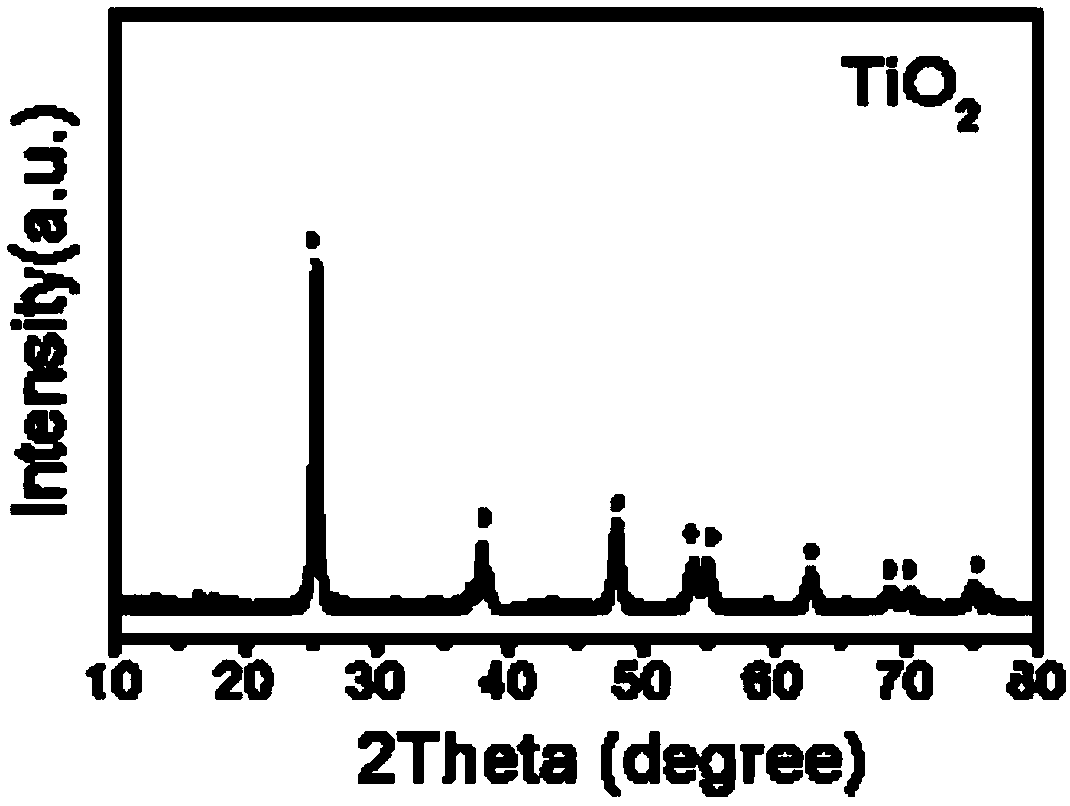
[0019] Add 0.025 mL of diethylenetriamine (EDTA) to 31.5 mL of isopropanol, and stir for 10 min. Then, 1.125 mL of diisopropyl di(acetylacetonato)titanate was added to the solution. Stirring was continued for 10 min. The resulting mixed solution was poured into a reaction kettle, and subjected to solvent heat treatment at 200° C. for 24 hours. After the reaction, the precipitate was washed three times with deionized water and absolute ethanol respectively, placed in an oven at 60° C., and dried for 24 hours to obtain an oxygen-vacancy titanium dioxide nanoflower material.
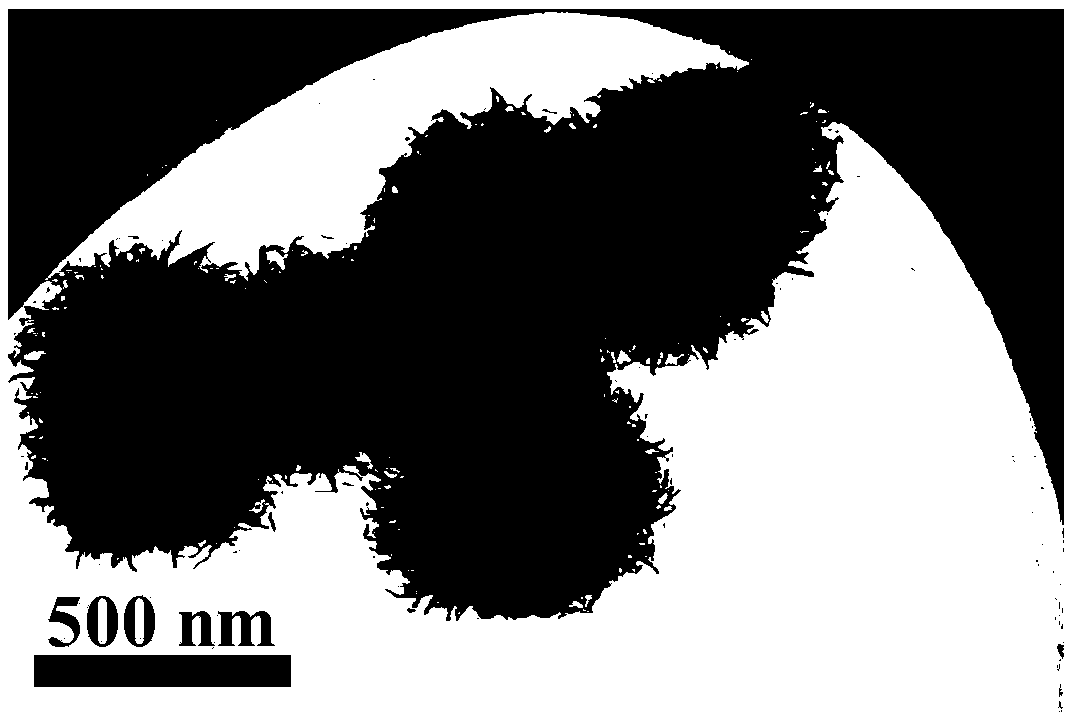
[0020] figure 1 , 2 They are scanning electron microscope images (SEM) and transmission electron microscope images (TEM) of the oxygen-rich vacancy titanium dioxide nanoflowers prepared in Example 1, respectively. It can be clearly seen from the figure that the size of the oxygen-rich titanium dioxide nanoflowers is 500-1000nm , which is formed by self-assembly of ultra-thin titanium dioxide nanosheets,...
Embodiment 2
[0024] Add 0.05 mL of diethylenetriamine (EDTA) to 31.5 mL of isopropanol solution, and stir for 10 min. 1.125 mL of diisopropyl di(acetylacetonato)titanate was added to the solution. Stirring was continued for 10 min. The resulting mixed solution was poured into a reaction kettle, and subjected to solvent heat treatment at 150° C. for 36 hours. After the reaction, the precipitate was washed three times with deionized water and absolute ethanol, placed in an oven at 60° C., and dried for 24 hours to obtain an oxygen-vacancy titanium dioxide nanoflower material.
[0025] Figure 5 Scanning electron microscope pictures (SEM) of the oxygen-vacancy titanium dioxide nanoflowers prepared for Example 2, as can be seen from the figure, the size of the titanium dioxide nanoflowers is 100-300nm, which is formed by the self-assembly of ultra-thin titanium dioxide nanosheets, nano The sheet thickness is 2-9 nm, and compared with the titanium dioxide nanoflowers obtained in Example 1, t...
Embodiment 3
[0027] Add 1.125 mL of diisopropyl di(acetylacetonate) titanate to 31.5 mL of isopropanol solution. Stir for 10 min. The resulting mixed solution was poured into a reaction kettle, and subjected to solvent heat treatment at 200° C. for 24 hours. After the reaction, the precipitate was washed three times with deionized water and absolute ethanol, placed in an oven at 60° C., and dried for 24 hours to obtain a sample.
[0028] Figure 6 For the scanning electron microscope picture (SEM) of the sample prepared for this example, it is obvious from the figure that diethylenetriamine is not added in the preparation process, and the prepared titanium dioxide does not form a hierarchical structure, but exists in the form of particles, indicating that The morphology and size of oxygen-vacancy-rich titania nanoflowers can be controlled by the amount of diethylenetriamine added.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com