4-piperidone compound as well as preparation method and application thereof
A technology of piperidone and compounds, which is applied in the field of preparation of antineoplastic drugs, can solve the problems of short action time and half-life, low bioavailability, etc., and achieve the effects of reducing dosage, enhancing drug efficacy, and strongly inhibiting activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
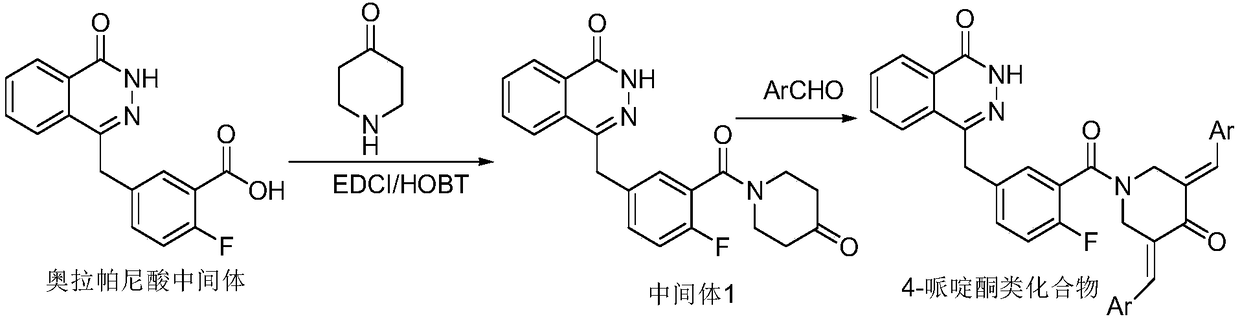
[0021] Preparation of N-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoyl]piperidin-4-one
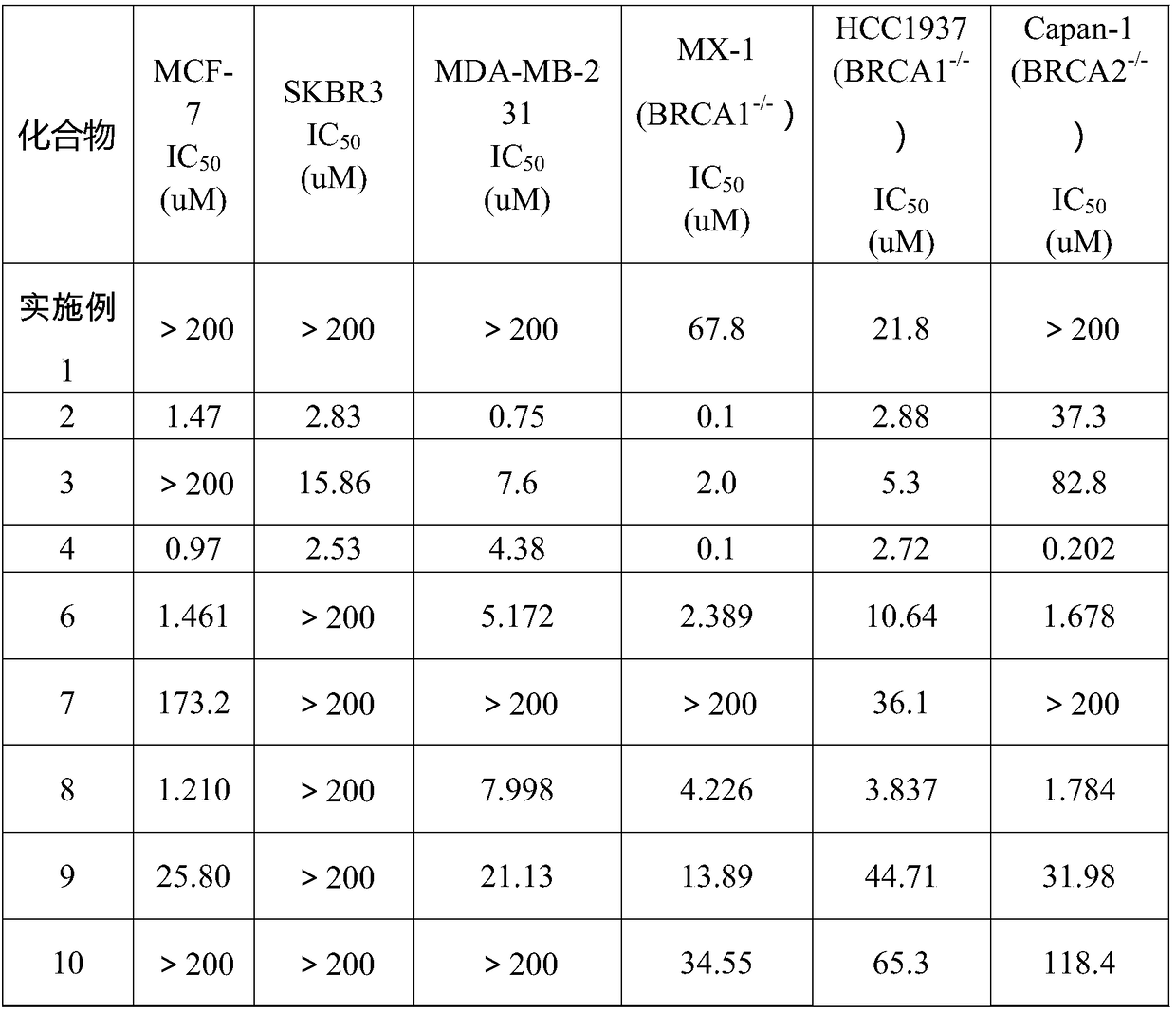
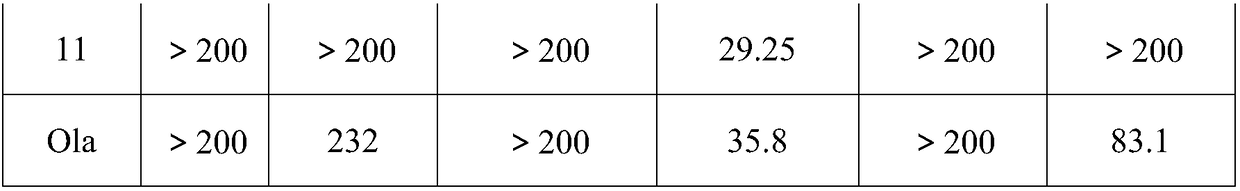
[0022] Dissolve olaparib acid intermediate (50mg, M=297.6, 0.168mmol), HBTU (82mg, M=379.24, 0.2184mmol), HOBT (6.8mg, M=135.12, 0.05mmol) in 5ml anhydrous THF (add 2ml DMF co-solvent), stir at room temperature for half an hour, DIPEA (0.86ml, 65.14mg, 0.504mmol, M=129.24, ), and 22.32 mg of 4-piperidone (M=148.05 hydrochloric acid form 0.1508 mmol), and stirred overnight at 50°C. Extracted with dichloromethane, washed 3 times with water and once with brine to obtain intermediate 1, namely N-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2- Fluorobenzoyl]piperidin-4-one.
[0023] Ethyl acetate:petroleum ether=3:1 column chromatography yielded 25.79mg. Yield 40%. Mp: 230.8-233.8℃.1HNMR(400MHz,Chloroform-d)δ10.94(s,1H),8.51(s,1H),7.81-7.76(m,3H),7.39(d,2H),7.09(m ,1H),4.33(s,2H),4.20-3.92(m,2H),3.63(s,2H),2.61(m,2H),2.46(s,2H).LC-MS(ESI)m / z :380.1[M + H] + .
Embodiment 2
[0025] 3,5-(E)-bis(2-pyridylmethenyl)-N-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluoro Preparation of Benzoyl]piperidin-4-one
[0026] Mix intermediate 1 55.28mg (0.146mmol, M=379.13), sodium hydroxide 1.67mg (0.02mmol, M=84.01), absolute ethanol 10ml, stir at room temperature, add 2-pyridinecarbaldehyde 44.6mg (0.4166 mmol, M=107.11), react at room temperature. Dichloromethane extraction, washed 3 times with water, washed with brine once. That is, 3,5-(E)-bis(2-pyridinemethenyl)-N-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2 -Fluorobenzoyl]piperidin-4-one. Ethyl acetate:petroleum ether=3:1 column chromatography yielded 20.79mg. Yield: 37%. Mp: 156.3-158.0°C. 1H NMR (400MHz, Chloroform-d) δ10.99(s,1H),8.79(s,1H),8.46(s,1H),8.18(s,1H),7.63(m,9H),7.38(d, 1H), 7.10(d, 3H), 6.81(s, 1H), 5.52(s, 2H), 5.24(s, 2H), 4.08(s, 2H). LC-MS (ESI) m / z: 558.2[ m + H] + .
Embodiment 3
[0028] 3,5-(E)-bis(3-pyridylmethenyl)-N-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluoro Preparation of Benzoyl]piperidin-4-one
[0029] This product was prepared from 55.28mg (0.146mmol, M=379.13) of intermediate 1 and 44.6mg (0.4166mmol, M=107.11) of 3-pyridinecarbaldehyde. The preparation method was the same as in Experimental Example 2, and 29.6mg of yellow solid was obtained. Yield 52%. Mp: 147.39°C-149.7°C. .1H NMR (400MHz, Chloroform-d) δ11.52(s,1H),8.78(s,1H),8.66(d,1H),8.62–8.52(m,1H),8.49(s,1H),8.41 (s,1H),7.88(s,2H),7.80(s,3H),7.72(s,2H),7.54(s,1H),7.44(s,1H),7.17(d,2H),6.80( s,1H),5.09(s,2H),4.63(s,2H),4.17(s,2H).LC-MS(ESI)m / z:556.21[M + H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com