Synthesis method of nicotinoyl hydrazone Schiff base compound as well as application of compound to bactericide
A technology of nicotinyl hydrazone Schiff base and synthesis method, which is applied in the direction of fungicides, botanical equipment and methods, applications, etc., and can solve the problem that the mechanism of acylhydrazone and its complexes is poorly understood, and the relationship between structure and activity is not very clear and other problems, to achieve the effects of easy industrialized large-scale application, easy separation, and low requirements for production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
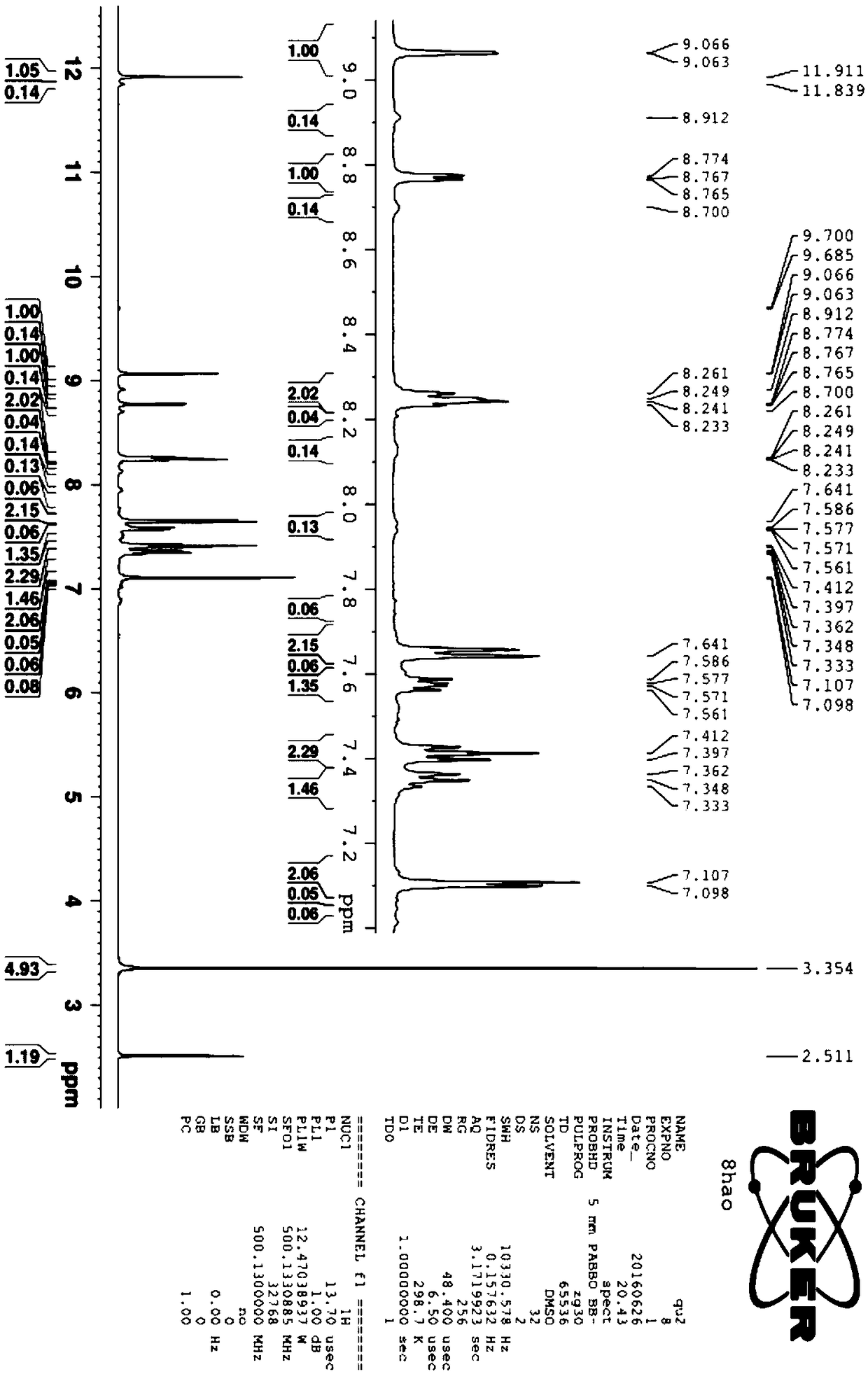
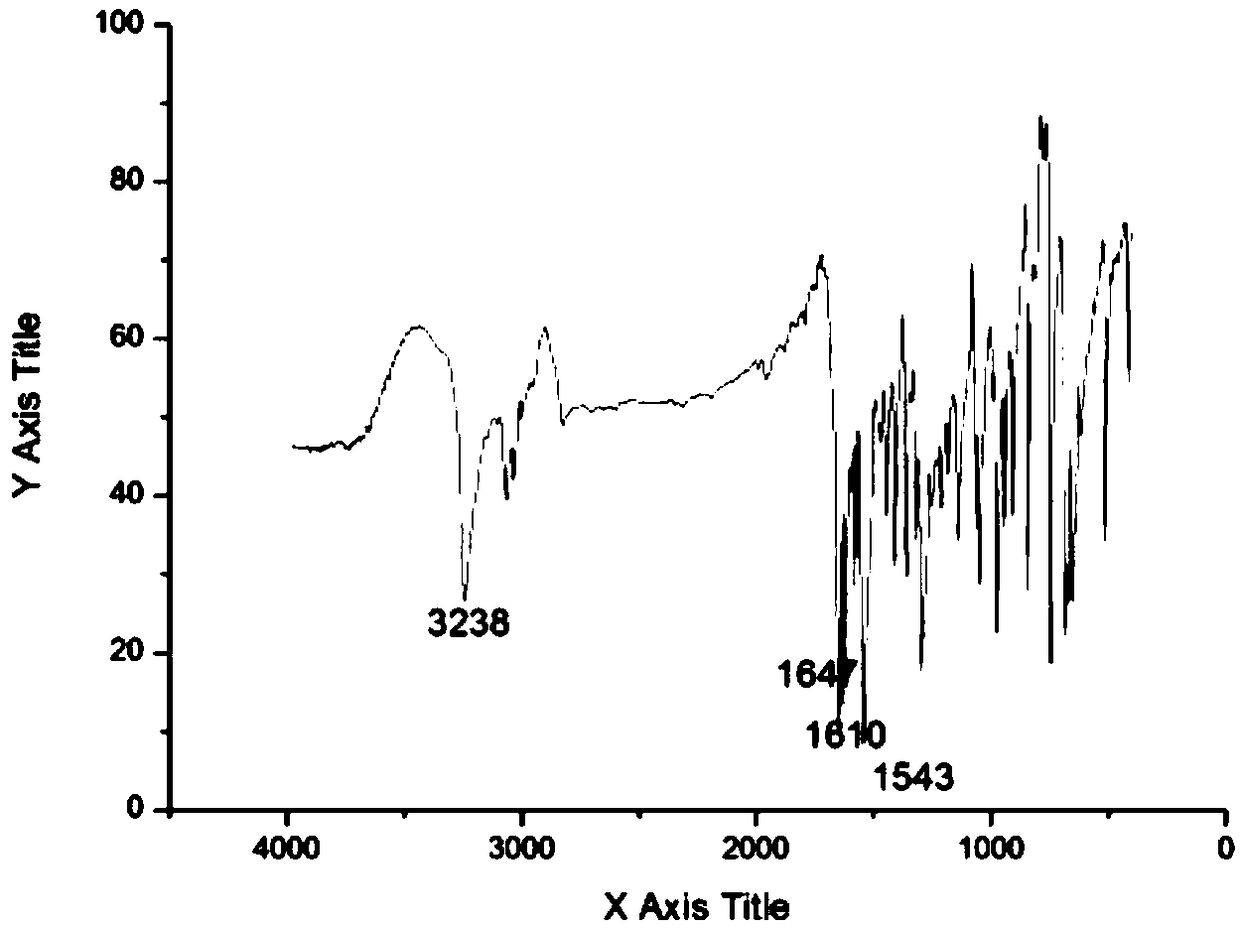
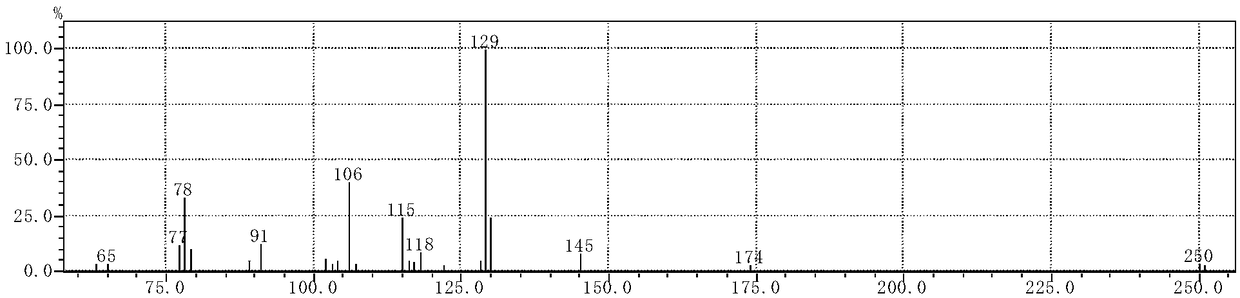
[0034] The synthetic method of the cinnamaldehyde nicotinyl hydrazone of the present embodiment, the method comprises the following steps:
[0035] (1) Synthesis of intermediate nicotinic acid hydrazide: 10 mL of hydrazine hydrate with a mass fraction of 80 wt % (0.165 mol) was dissolved in 50 mL of absolute ethanol, transferred to a 150 mL three-necked flask, and mixed uniformly. Add 20 mL (0.147 mol) of ethyl nicotinate dropwise through the dropping funnel (control the drop rate to 1 drop per second). Control the reaction temperature to 80°C, reflux and stir the reaction, and track it with TCL. After the reaction is over, let it stand and cool to room temperature. A white solid precipitates out and is filtered with suction to obtain the crude product 1. The crude product 1 is recrystallized with absolute ethanol. , vacuum-dried to obtain 12.658 g of white needle-shaped crystals (nicotinic acid hydrazide), with a yield of 79%.
[0036](2) Synthesis of the target compound: Di...
Embodiment 2
[0048] The synthetic method of the 1-naphthaldehyde nicotinyl hydrazone of the present embodiment, described method comprises the steps:
[0049] (1) Synthesis of intermediate nicotinic acid hydrazide: 10 mL of hydrazine hydrate with a mass fraction of 80 wt % (0.165 mol) was dissolved in 50 mL of absolute ethanol, transferred to a 150 mL three-necked flask, and mixed uniformly. Add 20 mL (0.147 mol) of ethyl nicotinate dropwise through the dropping funnel (control the drop rate to 1 drop per second). Control the reaction temperature to 80°C, reflux and stir the reaction, TCL tracking, after the reaction, let it stand and cool to room temperature, a white solid precipitates out, filter with suction to obtain the crude intermediate product, and recrystallize the crude product with absolute ethanol, After vacuum drying, 12.658 g of white needle-shaped crystals (nicotinic acid hydrazide) were obtained, with a yield of 79%.
[0050] (2) Synthesis of the target compound: Dissolve ...
Embodiment 3
[0062] The synthesis method of the pyridine-4-formaldehyde nicotinyl hydrazone of the present embodiment, the method comprises the following steps:
[0063] (1) Synthesis of intermediate nicotinic acid hydrazide: 10 mL of hydrazine hydrate with a mass fraction of 80 wt % (0.165 mol) was dissolved in 50 mL of absolute ethanol, transferred to a 150 mL three-necked flask, and mixed uniformly. Add 20 mL (0.147 mol) of ethyl nicotinate dropwise through the dropping funnel (control the drop rate to 1 drop per second). Control the reaction temperature to 80°C, reflux and stir the reaction, TCL tracking, after the reaction, let it stand and cool to room temperature, a white solid precipitates out, filter with suction to obtain the crude intermediate product, and recrystallize the crude product with absolute ethanol, After vacuum drying, 12.658 g of white needle-like crystals (nicotinic acid hydrazide) were obtained with a yield of 79%.
[0064] (2) Synthesis of the target compound: D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com