Monomethoxy polyethylene glycol modified ezetimibe and preparation method thereof
A technology of polyethylene glycol and monomethoxy, which is applied in the fields of organic chemistry, organic chemistry, etc., can solve problems such as the preparation method of ezetimibe without polyethylene glycol modification, and achieve increased stability in vivo and extended half-life , the effect of excellent lipid-lowering effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
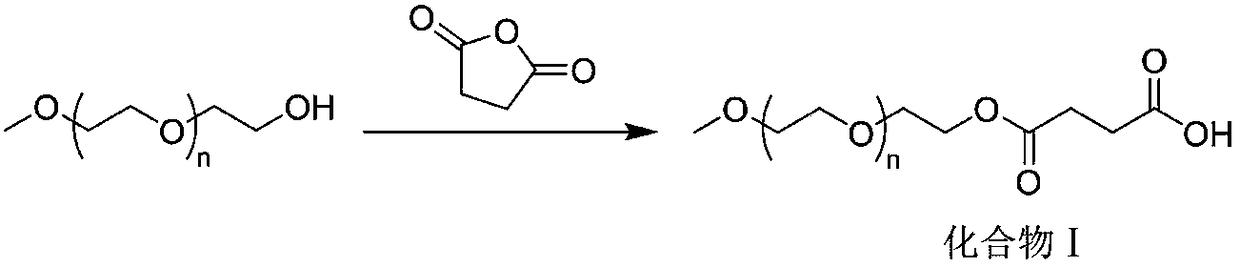
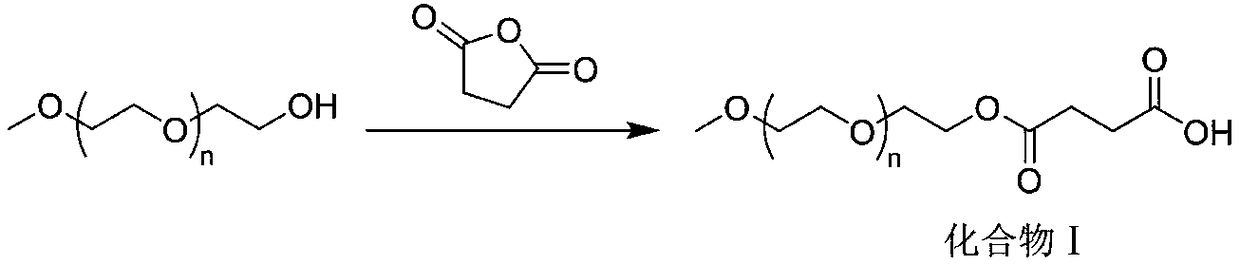
[0032] Example 1: mPEG 2 - Preparation of ezetimibe
[0033] 10 mmol of methoxydicondensed polyethylene glycol (mPEG 2 -OH) was dissolved in toluene, 20 mmol of succinic anhydride was added, and the reaction was stirred at 110° C. for 12 h. After the reaction was completed, the temperature was lowered, the solvent was spin-dried, water was added, and stirred at 60° C. for 2 h. Dichloromethane extraction, phase separation, the lower organic phase was obtained, dried over anhydrous sodium sulfate, filtered, spin-dried solvent, and recrystallized to obtain the product methoxydiethylene glycol butyrate (mPEG 2 -COOH), 9.1 mmol, yield 91%. 1 HNMR (400MHz, CDCl 3 ) NMR data are as follows: δ=4.24~4.20(m, 2H); δ=3.65~3.63(m, 2H); δ=3.54(s, 4H); δ=3.30(s, 3H); δ=2.85~2.82 (m, 2H); δ = 2.76-2.72 (m, 2H).
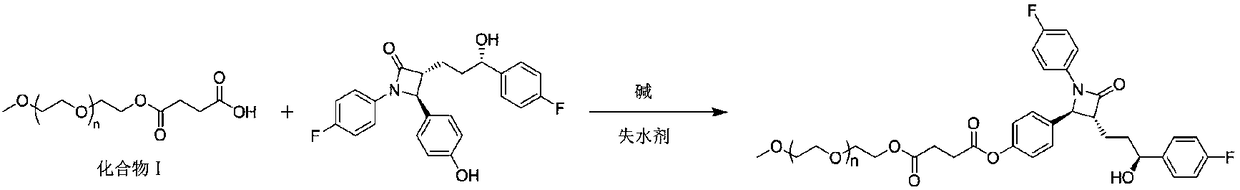
[0034] Obtained mPEG 2 8.5mmol of -COOH was dissolved in dichloromethane, 8.5mmol of DCC, 8.5mmol of DMAP, and 17mmol of ezetimibe were added in sequence, and the reaction was...
Embodiment 2
[0035] Example 2: mPEG 4 - Preparation of ezetimibe
[0036] 10 mmol methoxy tetracondensed polyethylene glycol (mPEG 4 -OH) was dissolved in toluene, 30 mmol of succinic anhydride was added, and the reaction was stirred at 110° C. for 12 h. After the reaction was completed, the temperature was lowered, the solvent was spin-dried, water was added, and stirred at 60° C. for 2 h. Dichloromethane extraction, phase separation, the lower organic phase was obtained, dried over anhydrous sodium sulfate, filtered, spin-dried solvent, and recrystallized to obtain the product methoxytetraethylene glycol butyrate (mPEG 4 -COOH), 9.2 mmol, yield 92%. 1 HNMR (400MHz, CDCl 3 ) NMR data are as follows: δ=4.26~4.21(m, 2H); δ=3.67~3.62(m, 2H); δ=3.53(s, 12H); δ=3.30(s, 3H); δ=2.86~2.82 (m, 2H); δ = 2.73-2.70 (m, 2H).
[0037] Obtained mPEG 4 -COOH 8.5mmol was dissolved in dichloromethane, 17mmol DCC, 17mmol DMAP, 17mmol ezetimibe were added in sequence, and stirred at 30°C for 24h. Aft...
Embodiment 3
[0038] Example 3: mPEG 12 - Preparation of ezetimibe
[0039] 10 mmol of methoxydodecylpolyethylene glycol (mPEG 12 -OH) was dissolved in toluene, 40 mmol of succinic anhydride was added, and the reaction was stirred at 110° C. for 12 h. After the reaction was completed, the temperature was lowered, the solvent was spin-dried, water was added, and stirred at 60° C. for 2 h. Dichloromethane extraction, phase separation, the lower organic phase was obtained, dried over anhydrous sodium sulfate, filtered, spin-dried solvent, and recrystallized to obtain the product methoxy dodecyl glycol butyrate (mPEG 12 -COOH), 9.4 mmol, yield 94%. 1 HNMR (400MHz, CDCl 3 ) NMR data are as follows: δ=4.25~4.21(m, 2H); δ=3.67~3.60(m, 2H); δ=3.54(s, 44H); δ=3.31(s, 3H); δ=2.86~2.80 (m, 2H); δ = 2.75-2.70 (m, 2H).
[0040] Obtained mPEG 12 -COOH 8.5mmol was dissolved in dichloromethane, 17mmol EDC, 17mmol triethylamine, 17mmol ezetimibe were added sequentially, and stirred at 30°C for 24h. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com