A kind of preparation method of pyridone derivatives and its intermediate
A pyridyl compound technology, applied in the field of preparation of cancer drugs, can solve the unfavorable industrial expansion of production, long reaction time, low yield and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0162] The following examples are used to further describe the present invention, but these examples do not limit the scope of the present invention.
[0163] The experimental methods not indicating specific conditions in the examples of the present invention are generally in accordance with conventional conditions, or in accordance with the conditions suggested by raw material or commodity manufacturers. Reagents without specific sources indicated are conventional reagents purchased in the market.
Embodiment 1
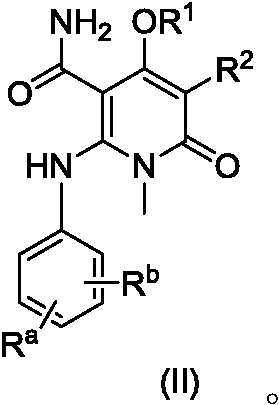
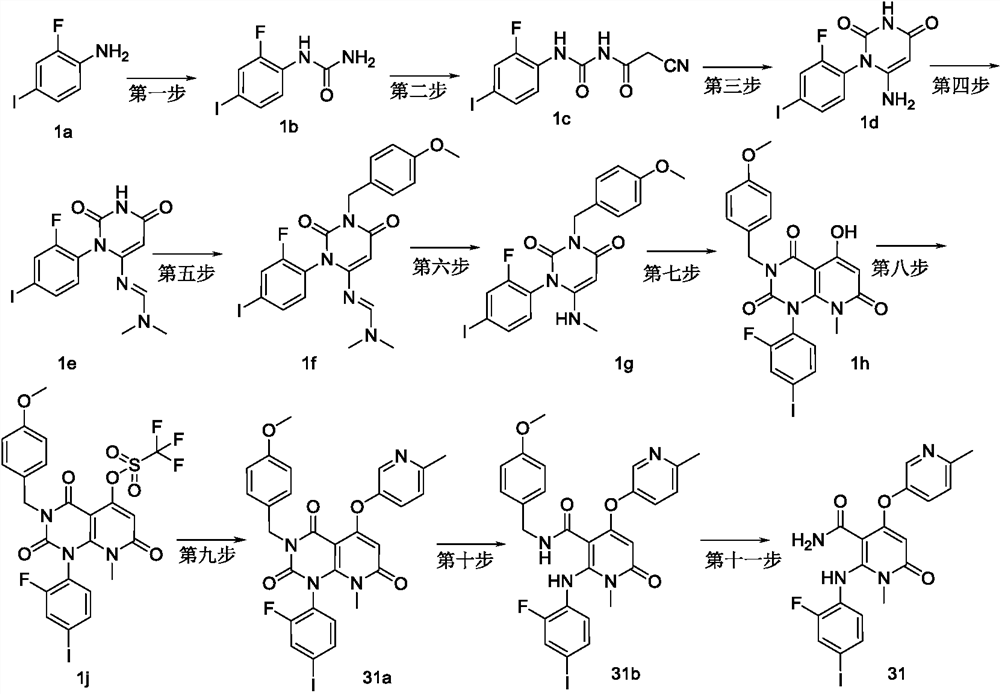
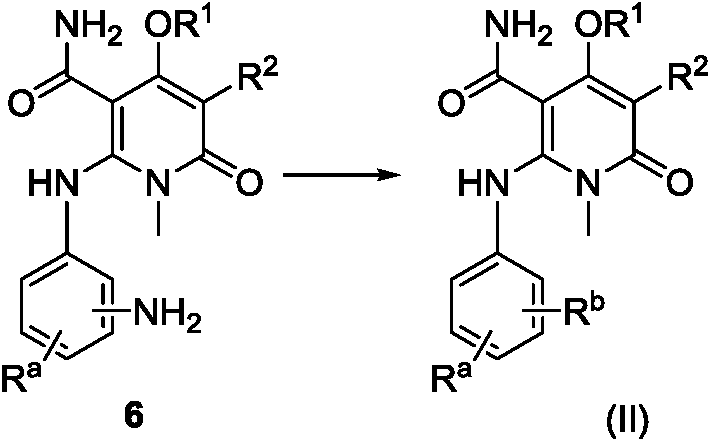
[0168] Example 1, 2-(2-fluoro-4-iodoanilino)-1-methyl-4-(6-methylpyridin-3-yloxy)-6-carbonyl-1,6-dihydropyridine - Preparation of 3-formamide (compound 31 or IIA)
[0169]
[0170] first step
[0171] 2-amino-4-hydroxy-1-methyl-6-carbonyl-1,6-dihydropyridine-3-carbonitrile (1-1)
[0172] Dimethyl malonate (39.6g, 0.3mol), malononitrile (19.8g, 0.3mol) and tetrahydrofuran (200ml) were added to the reaction flask, and 1,8- Diazabicyclo[5.4.0]undec-7-ene (DBU, 91.34g, 0.6mol), the dropwise addition was completed in 1 hour, stirred at room temperature for 18h, added dropwise with 30% methylamine aqueous solution (200ml), at room temperature Stir for 24h. Add sodium hydroxide solution (10N, 45ml) dropwise, stir at room temperature, react for 5h, add acetone and stir for 30min to filter under ice bath, collect the filter cake, dry under reduced pressure to obtain the title product (40g, light yellow solid), yield 80.8 %.
[0173] MS m / z(ESI): 166.2[M+1]
[0174] second step...
Embodiment 2
[0199] Example 2, 2-((2-fluoro-4-iodophenyl)amino)-1-methyl-4-((6-methylpyridin-3-yl)oxy)-6-carbonyl-1, Preparation of 6-dihydropyridine-3-carboxamide p-toluenesulfonate
[0200]
[0201] (1) Preparation of crude product
[0202] Drop into 2-((2-fluoro-4-iodophenyl)amino)-1-methyl-4-((6-methylpyridin-3-yl)oxy)-6-carbonyl-1 in the reaction bottle, 6-dihydropyridine-3-carboxamide (43g, 0.09mol), p-toluenesulfonic acid (19.0g, 0.10mol) and isopropanol (1.0kg), reflux for 2-2.5h. Stop heating, continue stirring for 12-14 h, stop the reaction, filter, wash the filter cake with isopropanol, and dry under reduced pressure to obtain the product (56.2 g, yield 97.0%, purity not less than 97% by HPLC).
[0203] (2) Purification of the product
[0204] Drop into 2-((2-fluoro-4-iodophenyl)amino)-1-methyl-4-((6-methylpyridin-3-yl)oxy)-6-carbonyl-1 in the reaction bottle, 6-dihydropyridine-3-carboxamide p-toluenesulfonate crude product (52.9g, 0.08mol), acetone (715g), purified water...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com