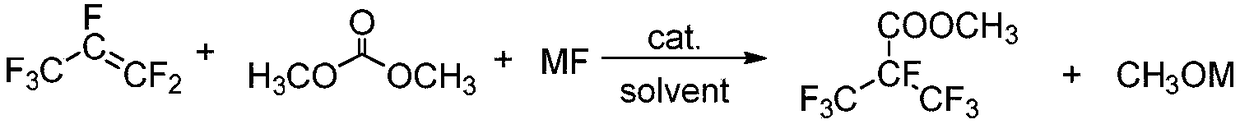
Preparation method for methyl heptafluoroisobutyrate
A technology of methyl heptafluoroisobutyrate and dimethyl carbonate, applied in the field of organic synthesis, can solve the problems of complicated operation, low intermediate reaction yield, uneconomical reaction, etc., and achieves the effects of high yield and simplified preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Under the protection of nitrogen replacement, 12.76g (0.22mol) of potassium fluoride, 19.81g (0.22mol) of dimethyl carbonate, and 100mL of acetonitrile were added in sequence to a 500mL dry autoclave, and slowly filled with 30.00% perfluoropropylene at -70°C. g (0.20mol), react at 70°C for 15h. After the reaction, the valve of the autoclave was opened in an ice-water bath, unreacted perfluoropropene was released slowly, the remaining reaction liquid was simply distilled, and the fraction at 35-37°C was collected to obtain 33.29 g of the product methyl heptafluoroisobutyrate, with a yield of 73.0%.
Embodiment 2
[0038] Under the protection of nitrogen replacement, 9.24g (0.22mol) of sodium fluoride, 19.81g (0.22mol) of dimethyl carbonate, and 100mL of acetonitrile were added sequentially to a 500mL dry autoclave, and slowly filled with 30.00% perfluoropropylene at -70°C g (0.20mol), react at 70°C for 15h. After the reaction was over, open the autoclave valve in the ice-water bath, slowly release the unreacted perfluoropropene, carry out simple distillation on the remaining reaction liquid, collect the fraction at 35-37°C, and obtain the product methyl heptafluoroisobutyrate 33.56g, the yield 73.6%.
Embodiment 3
[0040] Under the protection of nitrogen replacement, 12.76g (0.22mol) of potassium fluoride, 19.81g (0.22mol) of dimethyl carbonate, and 100mL of diethylene glycol dimethyl ether were successively added to a 500mL dry autoclave, and slowly Charge 30.00 g (0.20 mol) of perfluoropropylene, and react at 70° C. for 15 h. After the reaction, the valve of the autoclave was opened in an ice-water bath, unreacted perfluoropropene was released slowly, the remaining reaction liquid was simply distilled, and the fraction at 35-37°C was collected to obtain 29.77 g of the product methyl heptafluoroisobutyrate, with a yield of 65.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com