Preparation method, amplification primers and detection reagent for HPV full-length genome quality control product
A technique for amplifying primers and whole genomes, which is applied in biochemical equipment and methods, microbe determination/inspection, recombinant DNA technology, etc. It can solve the problems that cannot meet the performance evaluation of reagent methods, internal quality control, external quality evaluation, and low output. , more expensive and other issues, to achieve the effect of avoiding low connection efficiency, good applicability, and controllable concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
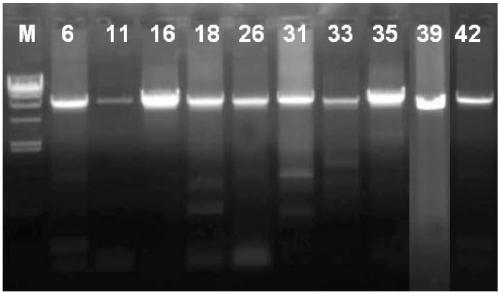
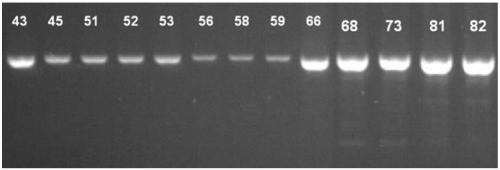
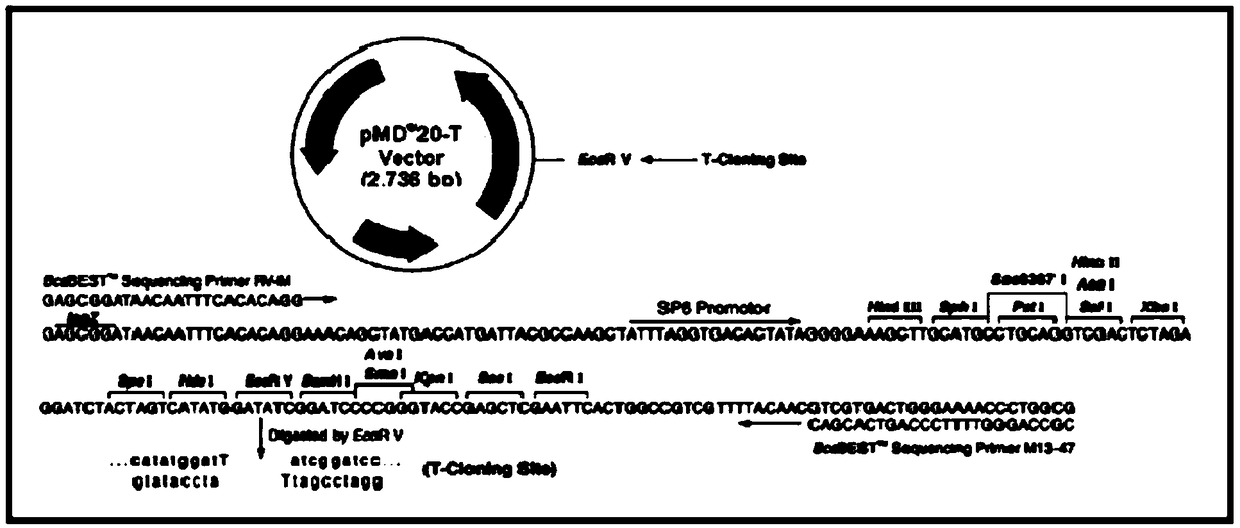
[0110] see Figure 1-8 , Figure 1-2 Electrophoresis results for electrophoresis of PCR products after sample DNA amplification; image 3 It is a schematic diagram of the structure of TVector (including the cloning site); Figure 4 Select the fluorescence profile for the sample, Figure 4 The middle curve corresponds to standard 1, sample, standard 2, standard 3 and standard 4 from left to right; Figure 5 is the standard curve formula; Image 6 The initial Ct value map for the stability study; Figure 7 Ct value change chart for stability research; Figure 8 It is the chart of the value determination results of 23 quality control substances by digital PCR absolute quantification method.
[0111] The preparation method of the human papillomavirus full-length genome quality control product of the present embodiment comprises:
[0112] (1) HPV nucleic acid extraction and type determination
[0113] Use the nucleic acid extraction reagent of Yaneng Biotechnology (Shenzhen)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com