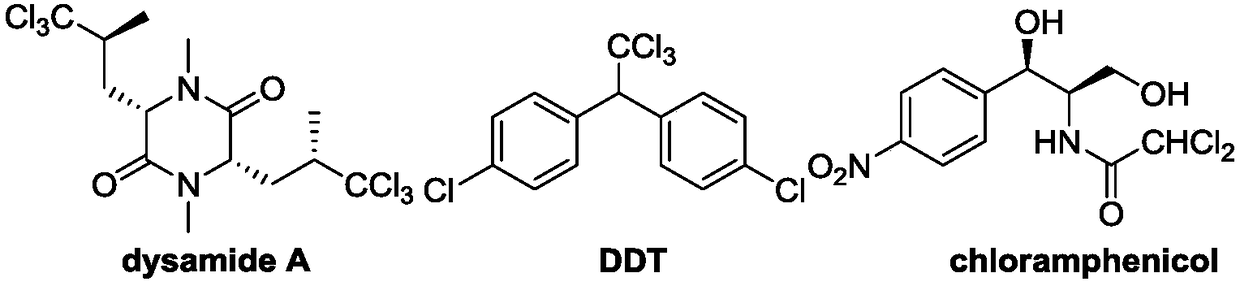
Polychloromethyl-substituted indoline compound and synthetic method and application thereof
A technology of polychloromethyl group and synthetic method, applied in the directions of organic chemistry method, organic chemistry, drug combination, etc., can solve the problems such as unreported research work, and achieve the effects of simple post-processing, convenient processing, and wide application range of substrates.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
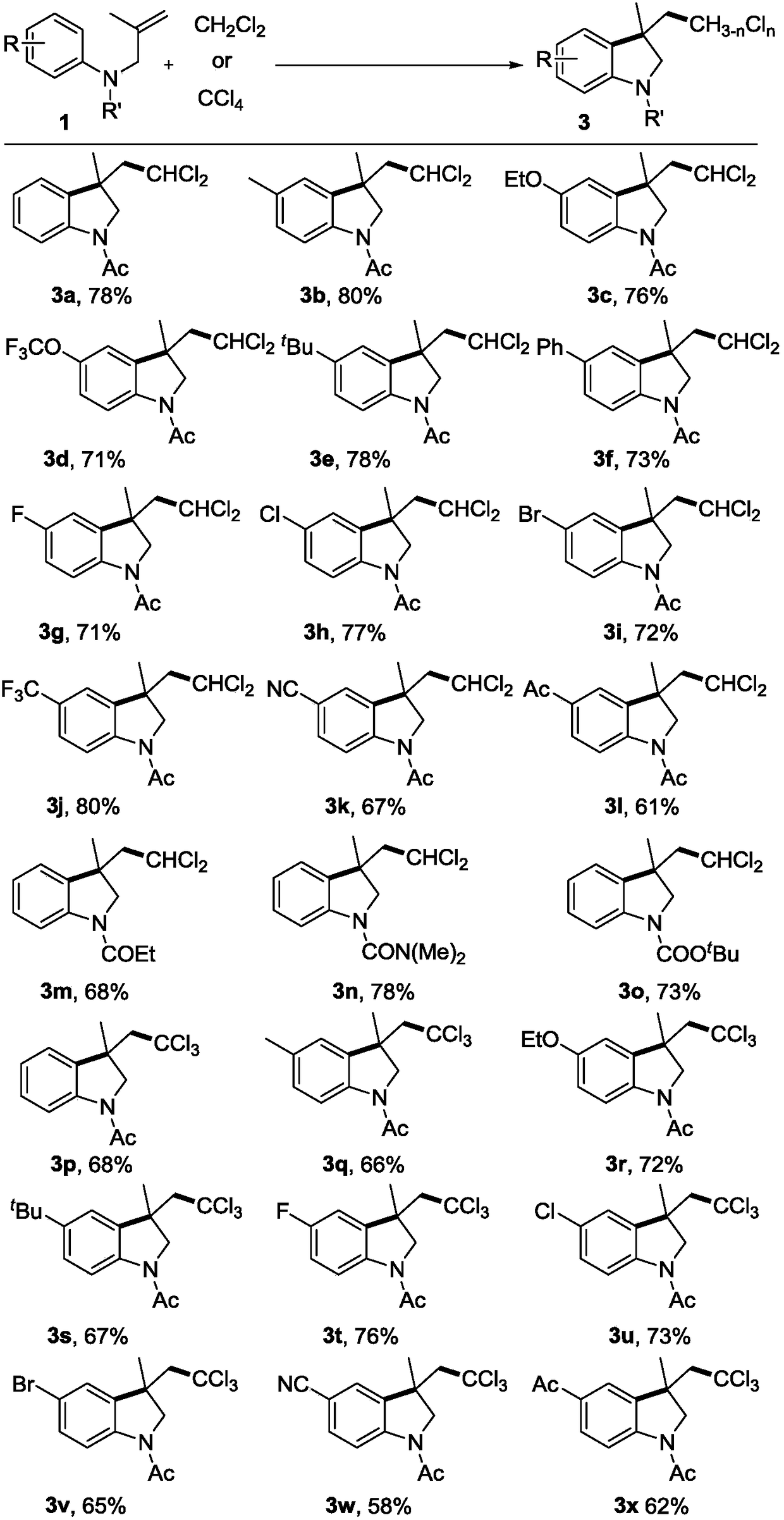
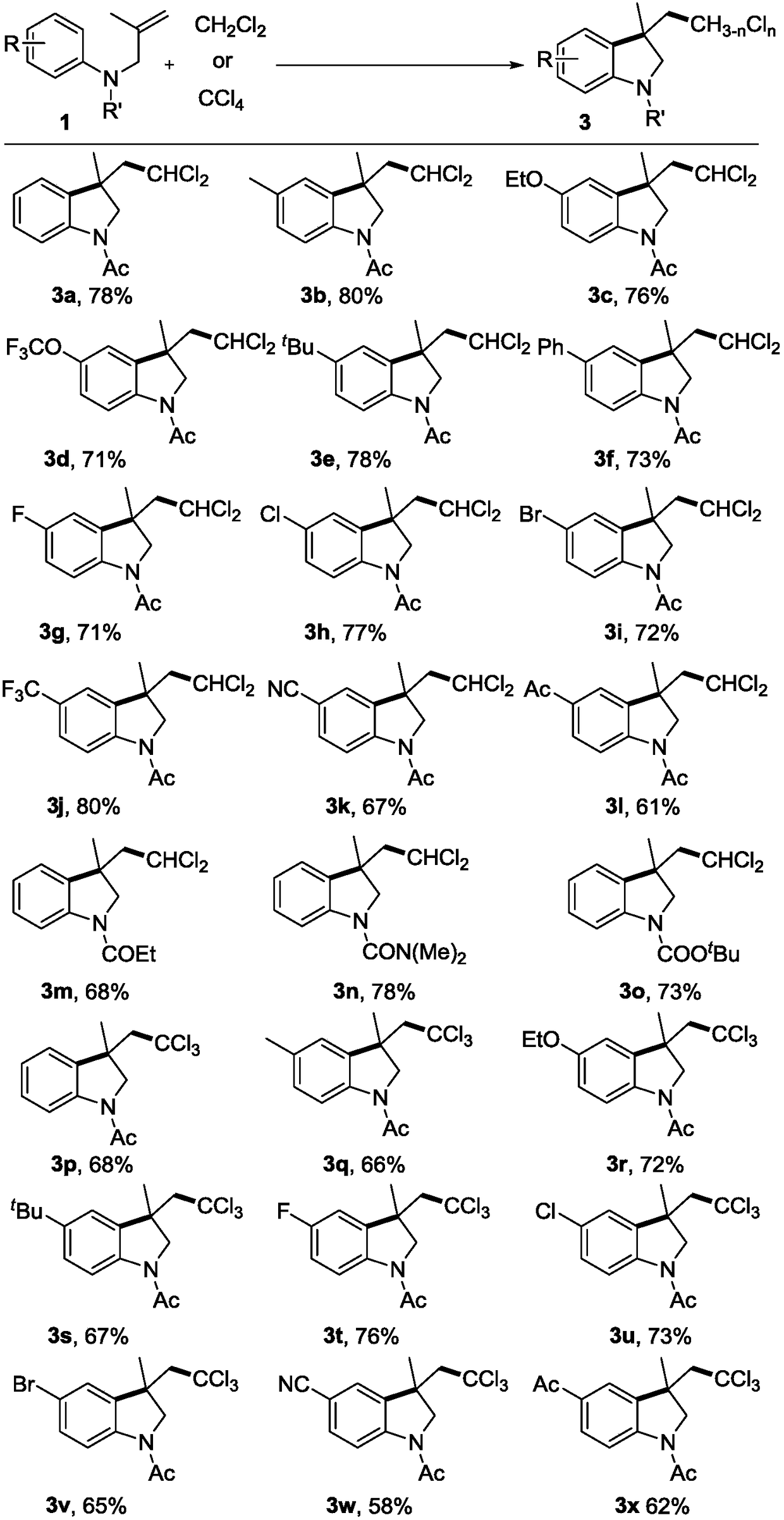
[0019] Under nitrogen protection, N-(2-methylallyl)-acetanilide 1a (0.2 mmol), di-tert-butyl peroxide (DTBP, 0.6 mmol), dichloromethane (2 mL) were added to a Schlenk reaction tube In, sealed. Heating to 100°C, the reaction time was 12 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3a was obtained by column chromatography with a yield of 78%.
Embodiment 2
[0021] Under nitrogen protection, N-(2-methylallyl)-acetyl-p-methylaniline 1b (0.2mmol), tert-butyl hydroperoxide (TBHP, 0.4mmol), dichloromethane (2mL) were added to Schlenk reaction tubes, sealed. Heating to 100°C, the reaction time was 24 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3b was obtained by column chromatography with a yield of 80%.
Embodiment 3
[0023] Under nitrogen protection, N-(2-methylallyl)-acetyl-p-ethoxyaniline 1c (0.2mmol), tert-butyl benzoyl peroxide (TBPB, 0.5mmol), dichloromethane (2mL ) into the Schlenk reaction tube and sealed. Heating to 120°C, the reaction time was 16 hours. After the reaction, the solvent was removed under reduced pressure, and the target product 3c was obtained by column chromatography with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com